A kind of β-1,6-glucanase and its coding gene and application
A glucanase and gene technology, applied in the field of agricultural microorganisms and plant protection, can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Purification and gene cloning of embodiment 1β-1,6-glucanase
[0068] 1. Separation and purification of 1β-1,6-glucanase
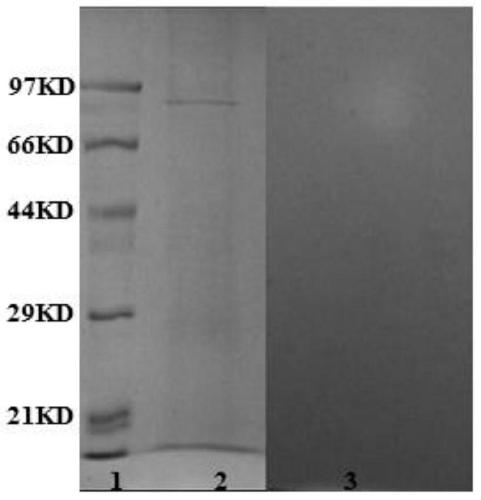
[0069] Inoculate the strain Corαllococcus sp.EGB (CCTCC NO: M2012528) into VY / 4 liquid medium (VY / 4 medium: 0.25% yeast cells, 0.1% CaCl 2 , pH 7.0), cultured on a shaker at 30°C for 2-3 days, collected the fermentation supernatant by centrifugation, concentrated the supernatant by 40%-80% ammonium sulfate gradient precipitation, passed through DEAE weak anion exchange column, hydrophobic column, sephardex G200 molecular sieve Combined with dextran substrate adsorption and desorption and other methods, the target protein is purified. By zymographic analysis (such as figure 1 ), determine that the band with a molecular weight of about 97KD on the SDS-PAGE protein electrophoresis is the target band.
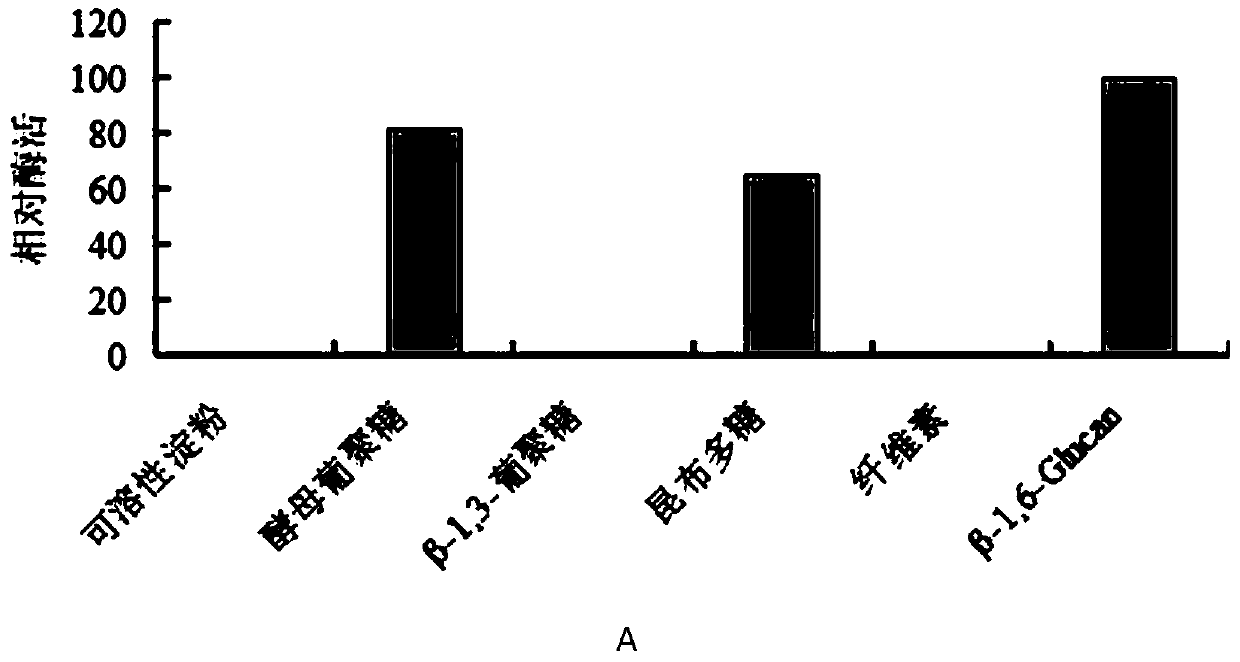
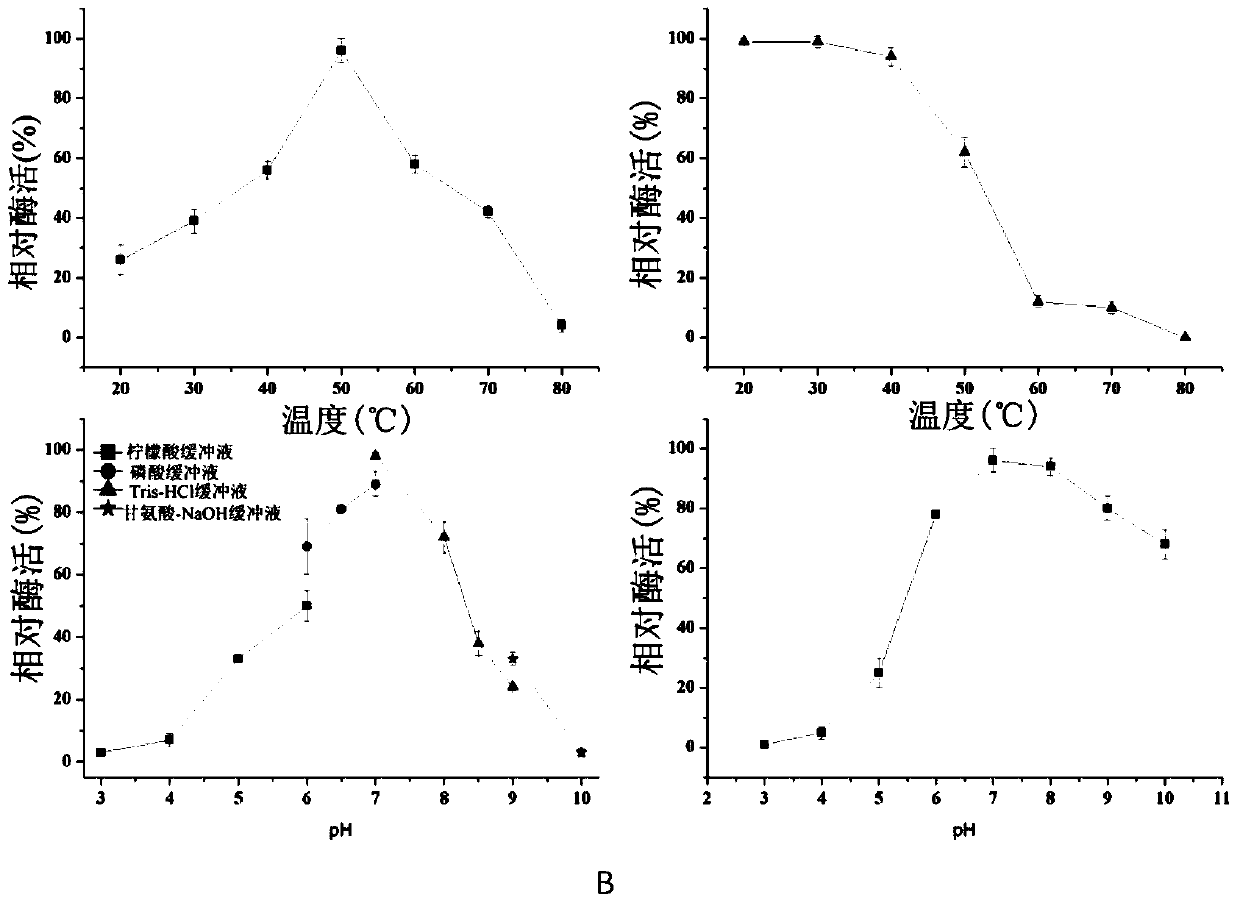
[0070] Yeast glucan, β-1,3-glucan, laminarin, agarin, glucan BIWP2 (doi:10.1016 / j.carbpol.2012.12.036), oat glucan, cellulose, wood Glycans were use...
Embodiment 2
[0089] Example 2. Heterologous expression of β-1,6-glucanase gene gluM
[0090] 2.1 Construction of expression vector gluM-pET-29a(+)
[0091] Digest the recombinant plasmid extracted in 1.3.3 and pET-29a(+) (Merck-Novαgen, Cat NO.69871) with NdeI and XhoI
[0093]
[0094] In a 37°C water bath, the enzyme digestion reaction was carried out overnight. The digested products were recovered by 0.75% agarose gel electrophoresis. The pET-29a(+) digested by the recovered fragment was enzyme-ligated to obtain the pET-29a(+) recombinant plasmid containing the β-1,6-glucanase gene.
[0095] The enzyme-linked pET-29a(+) recombinant plasmid containing β-1,6-glucanase gene was transformed into the expression host strain E.coli BL21(DE3) (NBE, Cat NO.C2527H) to obtain the recombinant microorganism E. coli BL21(DE3), spread LB plates containing 50mg / L kanamycin, pick a single colony to extract the plasmid and verify the gene sequence is correct by sequ...
Embodiment 3
[0099] Example 3. Functional verification of β-1,6-glucanase GluM
[0100] Use spore-forming medium to culture Magnaporthe grisea, and collect Magnaporthe grisea spores, which are derived from induced expression of recombinant β-1,6-glucanase GluM-BL21 (full-length gene of β-1,6-glucanase , gene sequence position: 79bp-3222bp, SEQ ID NO.1; amino acid sequence position: 27AA-1073AA, SEQ ID NO.2; remove signal peptide) and β-1,6-glucan derived from supernatant crude enzyme solution The enzyme GluM-S was co-cultured with blast spores on a hydrophobic membrane, and the germination of blast spore tubes and the formation of attached spores were observed at intervals. Simultaneously with bacterial strain supernatant crude enzyme liquid, supernatant enzyme liquid ultrafiltration liquid (molecular weight cut-off 10KD) and heat-inactivated enzyme liquid as contrast treatment, the result is as follows Figure 5 . The results showed that after 4 hours of treatment, compared with the nor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com