Enzymatic synthesis method of S-alpha-D-glucoside-L-cysteine
A technology of cysteine and glucoside, applied in the field of enzymatic synthesis of S-α-D-glucoside-L-cysteine, to achieve the effect of improving substrate utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
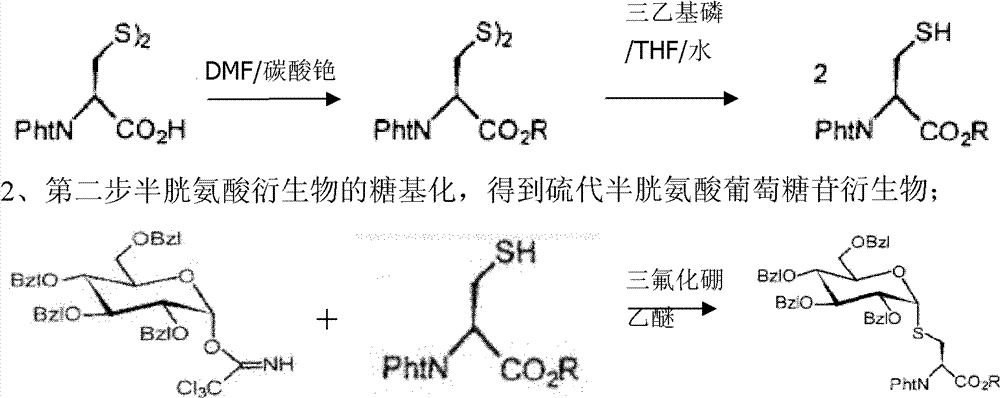
[0038] Embodiment 1 prepares and purifies sucrose phosphorylase
[0039] Bacteria: Leuconostoc mesenteroides ATCC12291
[0040] Seed activation medium: 10g / L peptone, 10g / L meat extract, 5g / L yeast extract, 20g / L glucose, 5g / L sodium acetate trihydrate crystals, 1ml / L Tween 80, 2g / L citric acid Triammonium, 2g / L K 2 HPO 4 , 20g / L agar, 0.2g / LMgSO 4 ·7H 2O, 0.05g / L MnSO 4 2H 2 O, pH5.4.
[0041] Fermentation medium: 20g / L sucrose, 10g / L soybean peptone, 10g / L yeast extract, 10g / LK 2 HPO 4 , 10ml / L Vitamin-mineral stock solution (1L contains VB 1 1g; VC 5g; anhydrous magnesium sulfate 40g; manganese sulfate monohydrate 30g; ferrous sulfate heptahydrate 1g), pH 7.6.
[0042] Sterilization conditions: Separately sterilize the glucose and metal ions in the seed activation medium; separately sterilize the sucrose in the fermentation medium, sterilize the Vitamin-mineral stock solution with a sterile filter, and sterilize the others by autoclaving at 121°C for 15 minutes. ...
Embodiment 2
[0048] Get 200 U of the sucrose phosphorylase obtained in Example 1, add 0.4 mol / L sucrose, 0.1 mol / L L-cysteine, 10 ml of MES buffer (pH 5.5, 10 mM), mix well and then stir The reaction was carried out for 13 hours, and the reaction temperature was 30°C. After the reaction, the enzyme was removed by microfiltration, and detected by Dionne U3000 detection system, and the conversion rate of L-cysteine was 5.2%.
Embodiment 3
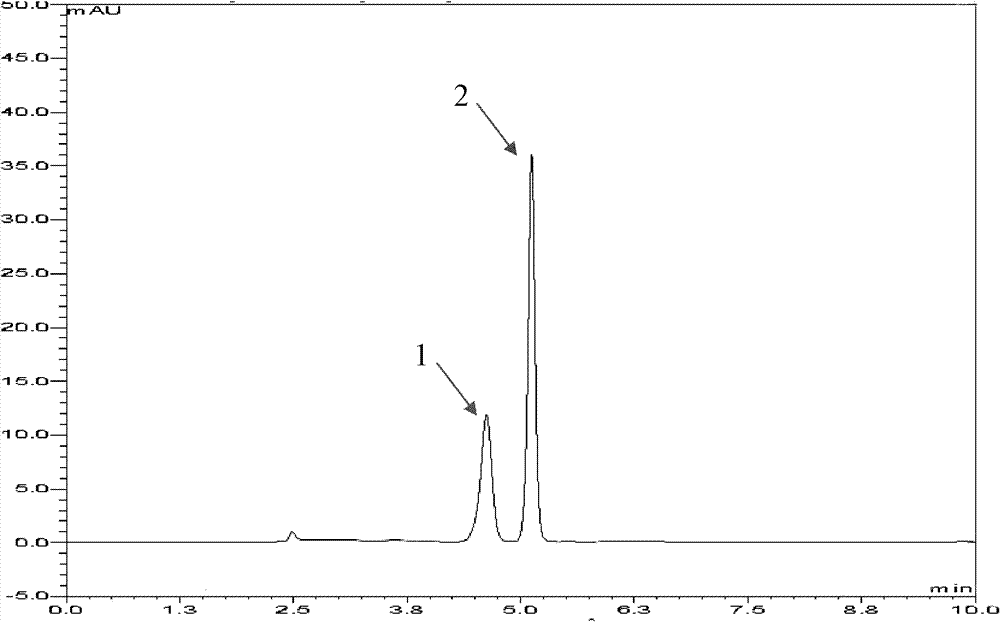
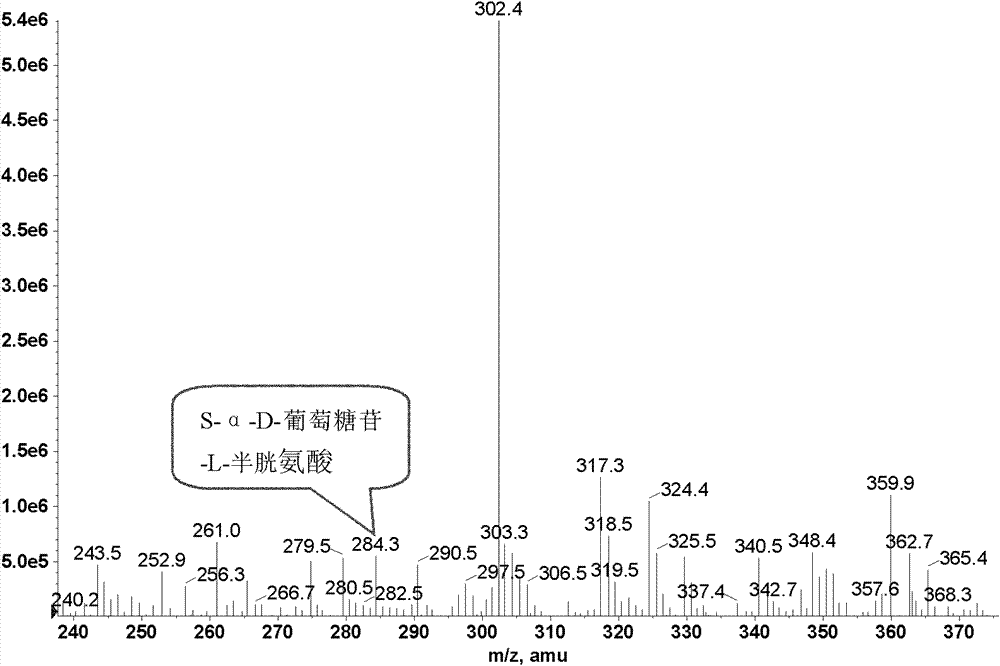
[0050] Get 200U of the sucrose phosphorylase obtained in Example 1, add to 0.5mol / L sucrose, 0.15mol / L L-cysteine, 10ml of MES buffer (pH7.0, 10mM), mix well and then stir The reaction was carried out for 12 hours, and the reaction temperature was 35°C. After the reaction, the enzyme was removed by microfiltration, and detected by Dionne U3000 detection system, and the conversion rate of L-cysteine was 10.1%. The liquid chromatogram is as figure 1 , take a sample to make a mass spectrum, the mass spectrum is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com