Glycosyltransferase gene and application thereof
A technology of glycosyltransferase and gene, which is applied in the field of genetic engineering to achieve the effect of reducing production cost and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Construction of marine silt metagenomic library and screening of positive clones
[0043] (1) Extraction of genomic DNA from marine sludge samples: Weigh 5 g of sample into a 50 mL centrifuge tube, add 13.5 mL
[0044] DNA extraction buffer, vigorously shake and mix, then add 100 μL proteinase K (10 mg / ml), invert repeatedly 5-6 times, then place in 37 °C water bath for 30 min, then add 1.5 mL 20% SDS, 65 °C water bath for 2 After centrifugation at 6,000 g for 10 min (upside down several times every 15 min), take the supernatant, extract twice with an equal volume of chloroform, centrifuge at 10,000 g for 20 min, take the supernatant, and add 0.6 times the volume of isopropyl Alcohol, left at room temperature for 1 h, centrifuged at 16,000 g for 20 min, discarded the supernatant, added 5 mL of pre-cooled 70% ethanol, centrifuged at 16,000 g for 5 min to collect the DNA precipitate, air-dried and dissolved with appropriate amount of TE buffer.
[0045] T...
Embodiment 2
[0056] Example 2 Glycosyltransferase gene Glyt7-2 Expression in E. coli
[0057] (1) PCR amplification of the glycosyltransferase gene: design primers according to the sequence of the above glycosyltransferase gene, and introduce a gene that can be inserted into the expression vector pET32a (+) (Novagen) Eco R I and Hin d III double restriction site, the primer sequence is as follows:
[0058] Glyt7-2 F: TGGCACCCGAATTCATGCGGATCGCGTTCCATAAGC
[0059] Glyt7-2 R: CCGTCGATAAGCTTTCATGCCGCGCCAATTGGGAAG
[0060] The extracted recombinant plasmid pUC19?lacZ– Glyt7-2 As a template, the above primers are used to carry out PCR amplification reaction, and the system is as follows:
[0061] pUC19?lacZ– Glyt7-2 template 5 ng 5×Buffer 1 μl dNTPs (2.5 mM) 4 μl Glyt7-2 F (20 μM) 1 μl Glyt7-2 R (20 μM) 1 μl PrimerSTAR (2.5 U / μl) 0.5 μl Make up to 50 μl with water
[0062] The PCR reaction conditions are as follows...
Embodiment 3
[0071] Example 3 Preparation and Purification of Recombinant Glycosyltransferase Glyt7-2 Crude Enzyme Solution
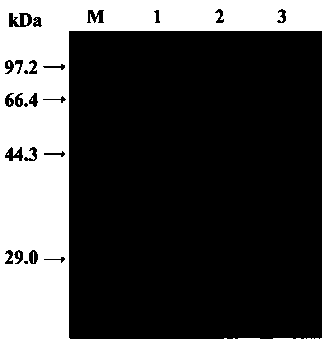
[0072] Inoculate the recombinant strains preserved in Example 2 in LB liquid medium containing 100 μg / ml ampicillin, and culture with vigorous shaking at 37°C until OD 600 =0.6~1.0, add the final concentration of 0.1~1.2 mM (IPTG), induce expression at 18~37 ℃ for 6~14 h, collect the bacteria, crush and centrifuge to obtain the crude enzyme solution. The crushed crude enzyme solution was purified with His·Bind Purification Kit (Novagen) and operated according to the instructions. SDS–PAGE electrophoresis analysis showed (attached figure 1 ), the purified recombinant glycosyltransferase is a single band with a molecular weight of about 64.5 kDa (of which 18 kDa is the fusion protein tag on the expression vector), which is consistent with the theoretically predicted protein molecular weight (46.5 kDa).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com