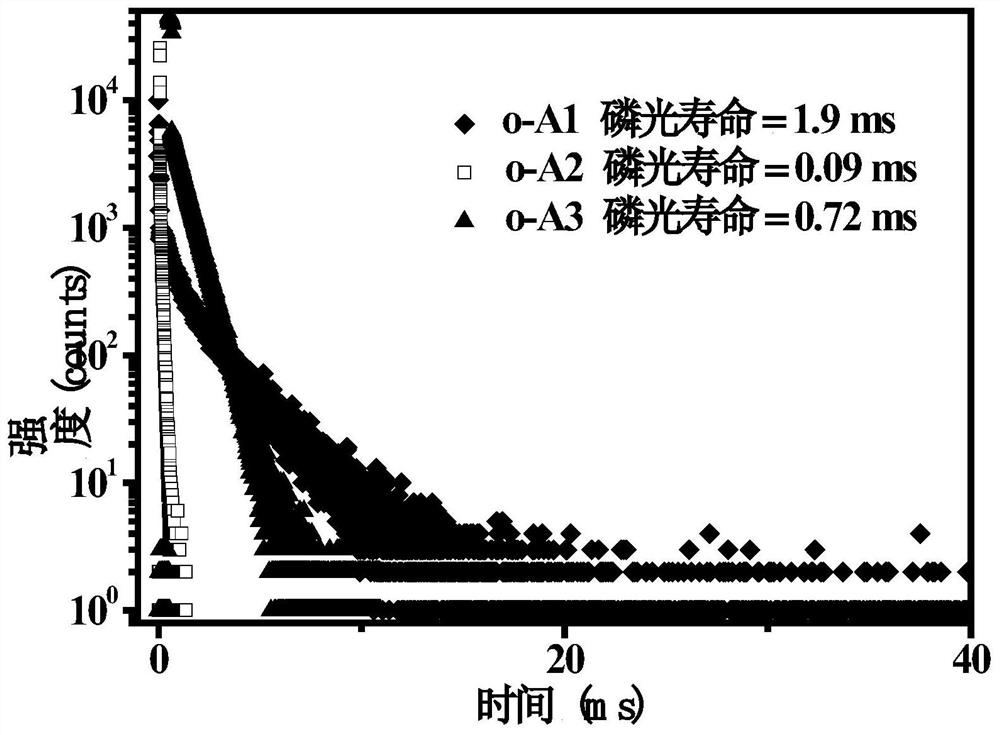
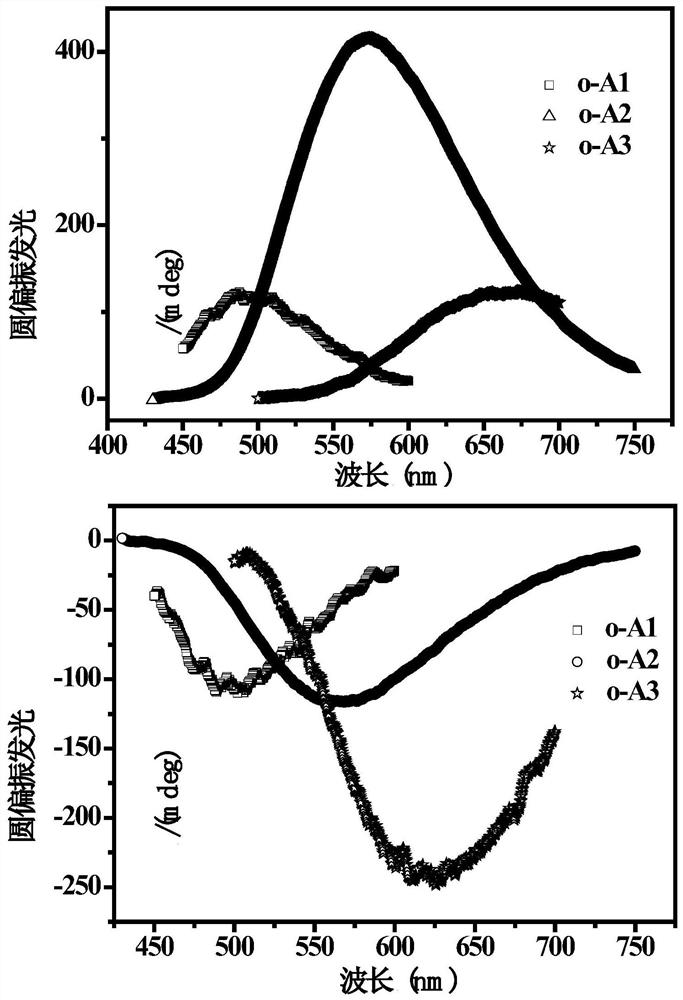
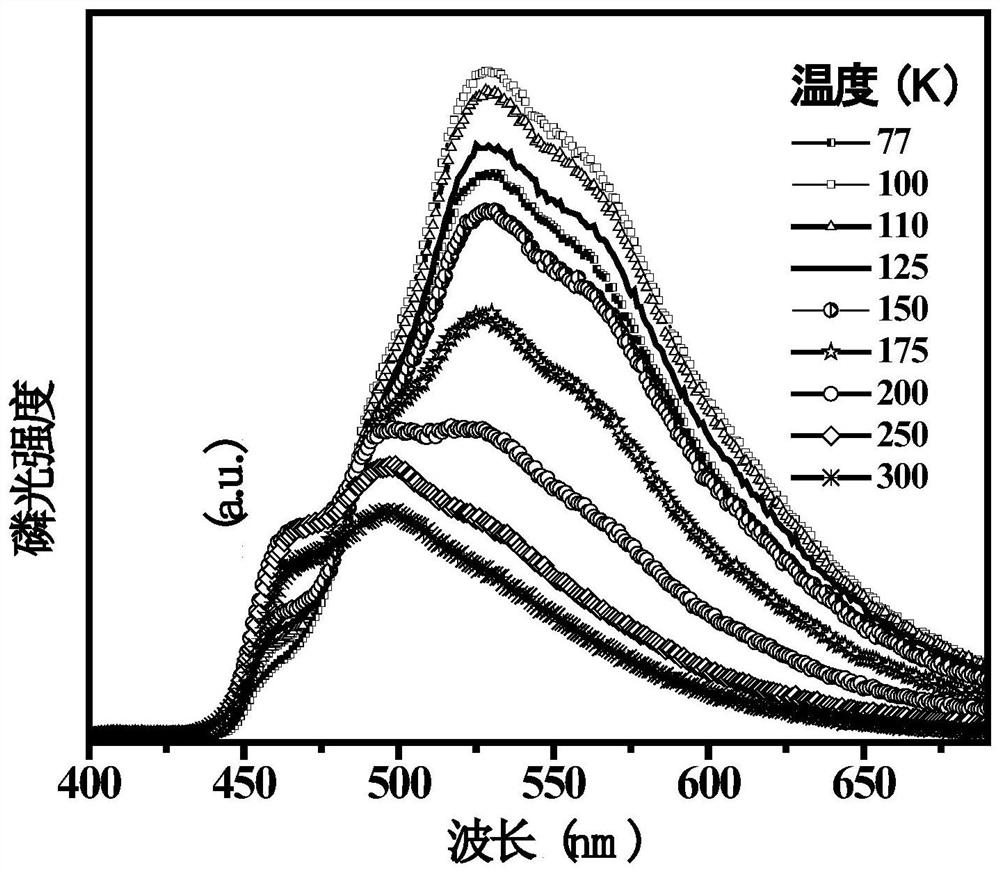
Fluorenyl-carborane photoelectric material as well as preparation method and application thereof
A technology of optoelectronic materials, carborane, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the fluorenyl-carborane photoelectric material of the present invention comprises the following steps:
[0034] Synthesis of o-A
[0035] Step 1: Anaerobic, anhydrous and protected from light, under the condition of 50-70°C, 9,9-dibutane-2-bromo-7-iodofluorene and aromatic terminal alkyne are dissolved in triethylamine solution, and stirred to react 4- 12h to obtain the intermediate compound A-R, the synthetic route is shown in the following formula:
[0036]
[0037] Step 2: at room temperature, decaborane (B 10 h 14 ) into the toluene solution dropwise, heated to 90-120°C, refluxed for 2-4h, cooled to 20-50°C, then added intermediate compound A-R, and the temperature rose to 90-120°C Reflux for 2-12h to prepare the target compound o-A. The synthetic route is shown in the following formula:
[0038]
Embodiment approach
[0040] The present invention designs and synthesizes a fluorenyl-carborane photoelectric material with an asymmetric central structure type, and the aryl R-substituted functional group of the carborane is any one of benzene, benzocarbazole, triphenylamine, naphthalene, anthracene, pyrene, etc. .
[0041] 1. The specific implementation method is as follows:
[0042] Preparation of o-A1:
[0043] The synthetic route is as follows:
[0044]
[0045] Step 1: Weigh 9,9-dibutane-2-bromo-7-iodofluorene (6.00g, 12.42mmol), an appropriate amount of 5% copper iodide CuI catalyst, and tetrakistriphenylphosphine palladium catalyst to avoid light Under the protection of nitrogen, add it to a 250mL double-necked round bottom flask, vacuumize and change the nitrogen at least three times, and add the anhydrous and oxygen-free triethylamine (Et 3 N) 60mL, add phenylacetylene (1.50mL, 13.66mmol, 0.93g / mL) dropwise under the condition of heating and stirring in an oil bath, raise the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com