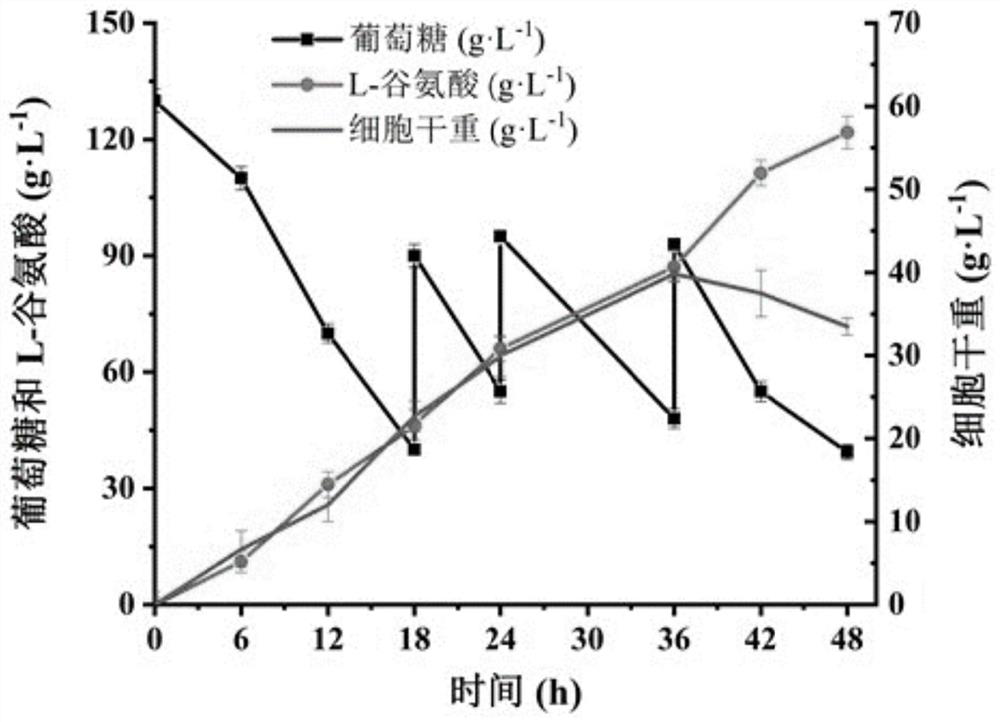
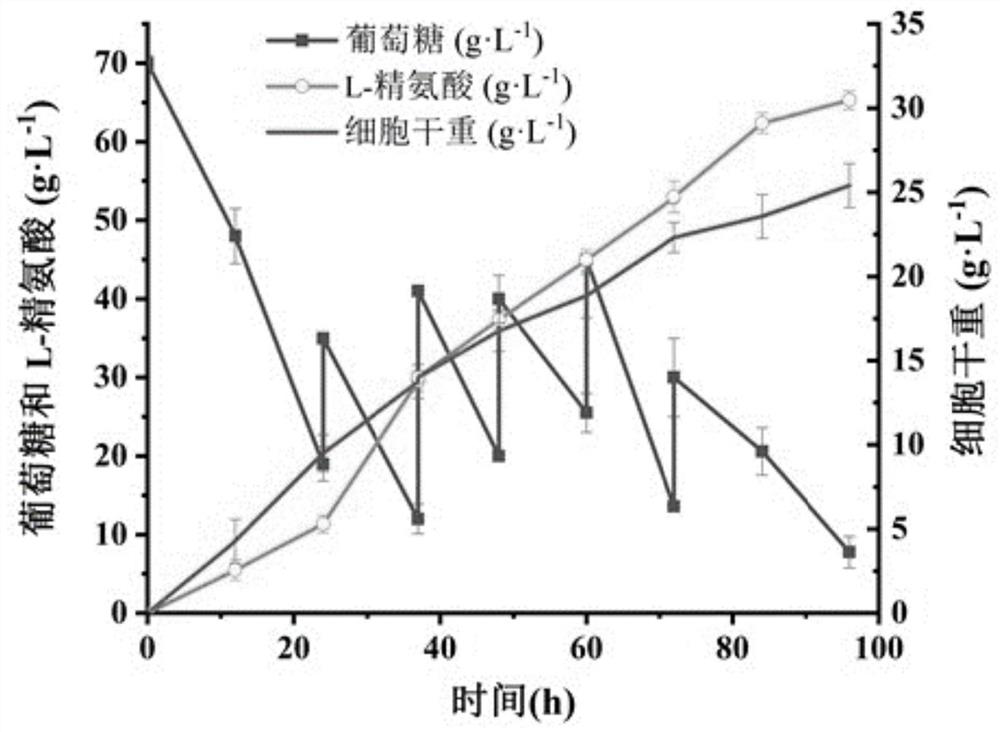
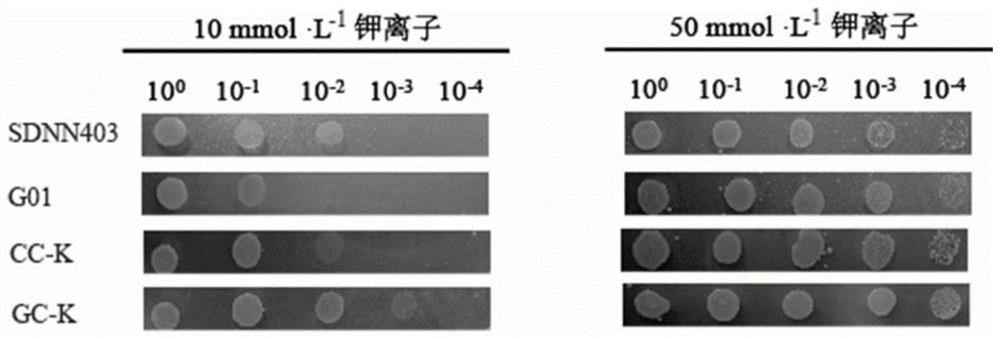
Method for promoting corynebacterium glutamicum to synthesize amino acid by using ion transport protein
A technology of Corynebacterium glutamicum and amino acids, which is applied in the field of bioengineering and can solve problems such as limiting the high output of high-value products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Construction of recombinant bacteria overexpressing functional potassium uptake protein CglK
[0026] Using the genomes of Corynebacterium glutamicum G01 and Corynebacterium blunt-toothed SDNN403 as templates respectively, using primers 10-cglKctapK-1 / 2 (nucleotide sequence as shown in SEQ ID NO.5 and SEQ ID NO.6) and 10 - cglKctapK-3 / 4 (nucleotide sequences shown in SEQ ID NO.7 and SEQ ID NO.8) respectively amplified upstream cglK and downstream ctapK gene fragments. After recovery, the above and downstream fragments were used as templates, and the tandem gene cglKctapK fragment was amplified by fusion PCR using the primer pair 10-cglKctapK-1 / 4. The pDXW-10 plasmid was purified and recovered by digestion with BamHI and HindIII enzymes, and the recovered product was ligated with the cglKctapK fragment using homologous recombinase to obtain a ligation product. The ligated product was heat-shocked to transform E.coliBL21 competent, spread it on a kanamycin-res...
Embodiment 2
[0028] Embodiment 2: Determination of bacterial strain growth under different potassium ion concentrations
[0029] Pick a circle of plate-activated strain GC-K and strain CC-K into liquid LBG medium, culture at 30°C, 180r / min rotary shaker until OD 600 ≈10.0 as seed solution. Take 1mL seed solution and transfer to 25mL L in a 250mL shaker flask 0 The basic medium was starved for 2-3 hours, the cells were washed with sterile PBS, fully suspended, centrifuged at 6000r / min for 10min, the supernatant was discarded in an ultra-clean bench, washed and centrifuged three times repeatedly, and the precipitate was retained, and finally washed with PBS (purchased from Thermo Fisher) suspension to OD 600 ≈1.0, then dilute 10 with sterile water -1 、10 -2 、10 -3 、10 -4 Take 1 μL of different gradients of bacterial solution at different potassium ion concentrations (10mmol / L and 50mmol / L respectively) and pH 7.0 for spot plate experiments, culture at 30°C, and closely observe the grow...
Embodiment 3
[0030] Example 3: Determination of Intracellular Pyruvate Kinase Activity
[0031] Take the sample fermented for 24 hours in a shaker flask, centrifuge and discard the supernatant, add 1mL extract for every 5 million cells, break the cells with ultrasonic waves, take the supernatant, and put it on ice for testing. Add the reagent according to the instructions of the Solebol-Pyruvate Kinase Activity Detection Kit, and record the change of the absorbance value of each tube at 340nm. The activity of pyruvate kinase was calculated according to the formula in the manual. The activity of pyruvate kinase in the mutant strain overexpressing the functional potassium uptake protein CglK and the cation transporter CTAPK was significantly higher than that of the original strain.
[0032]The pyruvate kinase activities of the strains GC-K and CC-K were 0.85U / mL and 0.89U / mL respectively, and the pyruvate kinase activities of the starting strains Corynebacterium glutamicum G01 and Corynebac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com