Highly active T cell in-vitro culture kit and culture method
A kit and high-activity technology, applied in the direction of cell culture active agent, culture process, tissue culture, etc., can solve the problems of increasing operator's operation time and cell culture cycle, increasing the use of reagent consumables, increasing the risk of experimental operation, etc., to achieve Reduce pain and medical costs, increase consumption, and have no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
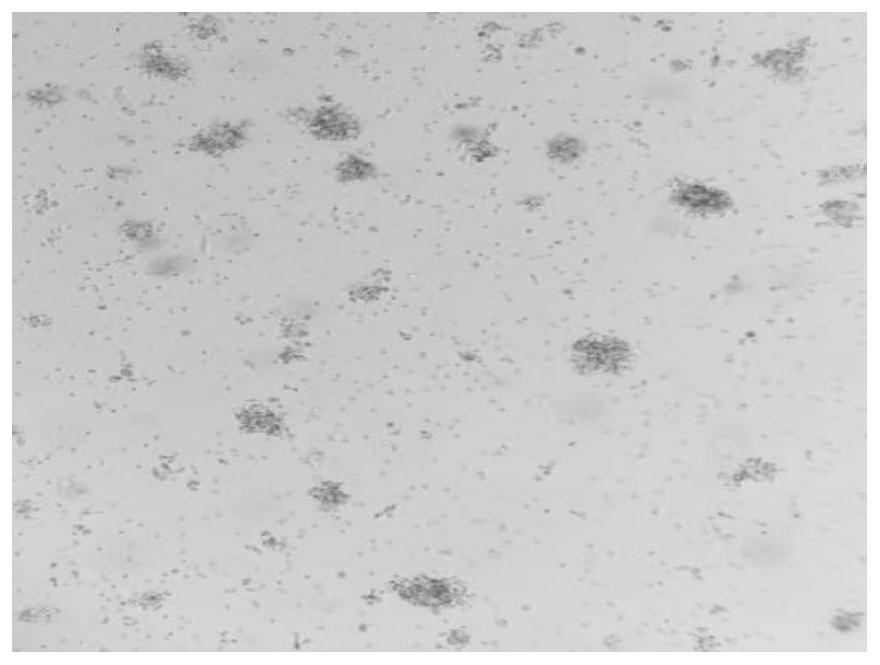
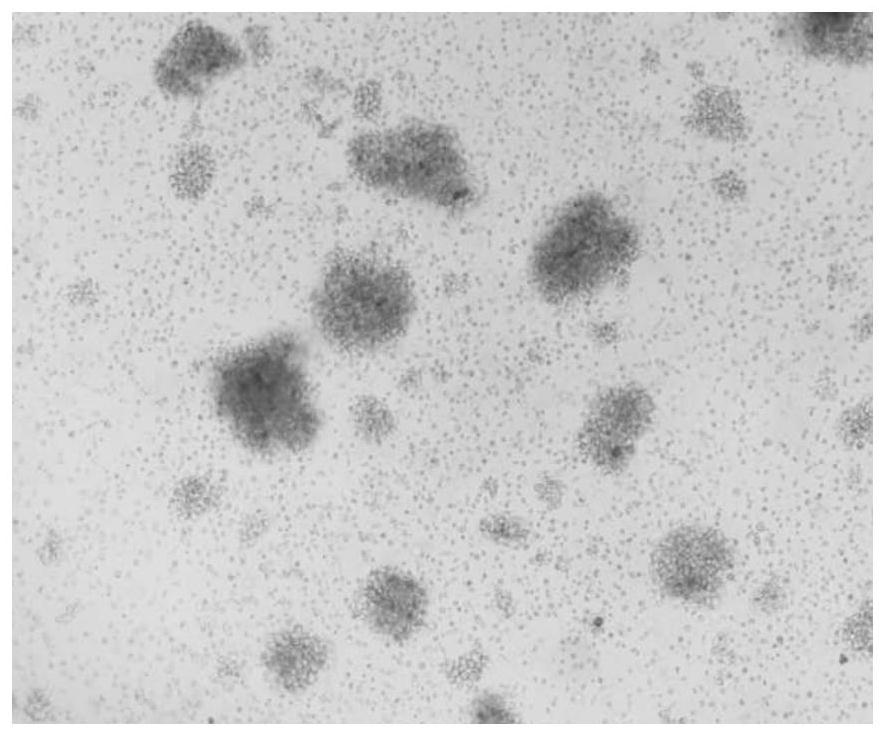
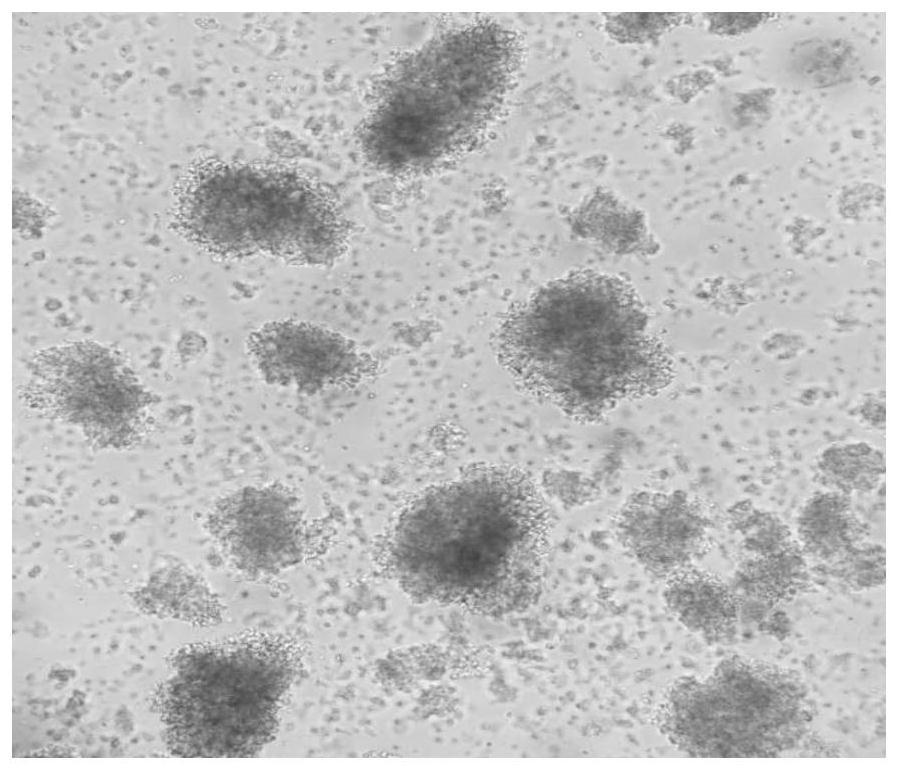
Image
Examples
Embodiment 1
[0047] This example is used to introduce a kit for in vitro culture of highly active T cells of the present invention and its preparation and quality testing of the kit. The highly active T cells (HAT) of the present invention specifically refer to the γδT and CIK cells. In combination, the in vitro culture kit of the present invention can facilitate the simultaneous cultivation of two kinds of cells (γδT cells and CIK cells) at one time in clinical practice, reduce the risk of experimental operation, shorten the operation time of operators and cell culture cycle, and effectively reduce the cost of reagent consumables use.
[0048] A high-activity T cell in vitro culture kit, including the main components of zometa (zoledronic acid), PHA (phytohemagglutinin, phytohemagglutinin), her-2 (human epidermal growth factor receptor 2, human epidermal growth factor receptor 2) , INF-γ (Interferon-γ) induction solution, the main component is IL-2 (Interleukin 2) stimulating solution, the m...
Embodiment example 1
[0084] Implementation case 1 results:
[0085] Test items Inducer Stimulant Activation solution Amplification solution determination skills requirement Endotoxin<0.06EU / ml
<0.06EU / ml
<0.06EU / ml
<0.06EU / ml
qualified<0.25EU / ml
Mycoplasma Negative Negative Negative Negative qualified Negative Fungus Negative Negative Negative Negative qualified Negative bacterial Negative Negative Negative Negative qualified Negative
[0086] Table 1
[0087] As another realization, a high-activity T cell in vitro culture kit includes an induction solution with main components of zometa, PHA, her-2, and INF-γ, a stimulating solution with IL-2 as the main component, and IL -2. The activation solution of IL-1α and IL-7, the main components of which are the amplification solution of IL-2 and IL-21; in the induction solution, the effective concentrations of zometa, PHA, her-2, and INF-γ are respectively 5ug / ml, 1ug / ml, 10ug / ml and 100IU / ml; in the stimulation solution, the effective concent...
Embodiment 2
[0090] Collect human peripheral blood from a healthy volunteer, separate mononuclear cells, culture them with the kit for highly active T cells (γδT and CIK cells) described in Example 1 of the present invention, and analyze the number of cell expansion, cell viability and cell proliferation multiples .
[0091] The culture method of the present invention includes the following steps:
[0092] 1. Preparation of PBMC (peripheral blood mononuclear cells): 40 ml of human peripheral blood was collected using a heparin sodium anticoagulation tube, and the blood was separated according to density gradient centrifugation. Separate the plasma and mononuclear cells in sequence, and inactivate the plasma at 56°C for 30 minutes and centrifuge for use to prepare mononuclear cells: Dilute the separated plasma cells with PBS (phosphate buffered saline) at a ratio of 1:1, and inject them into lymphocytes Slowly rise and fall on the separating fluid by centrifugation, and carefully aspirate monon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com