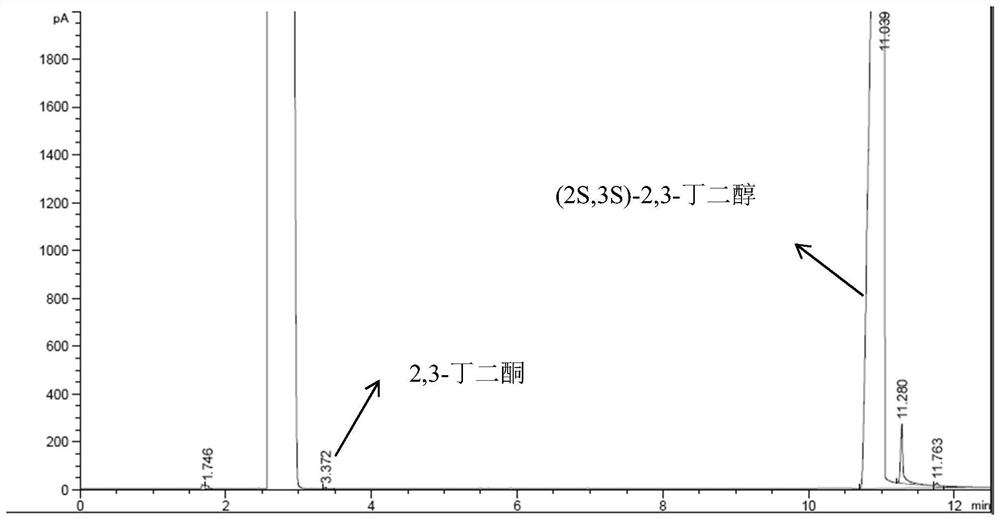
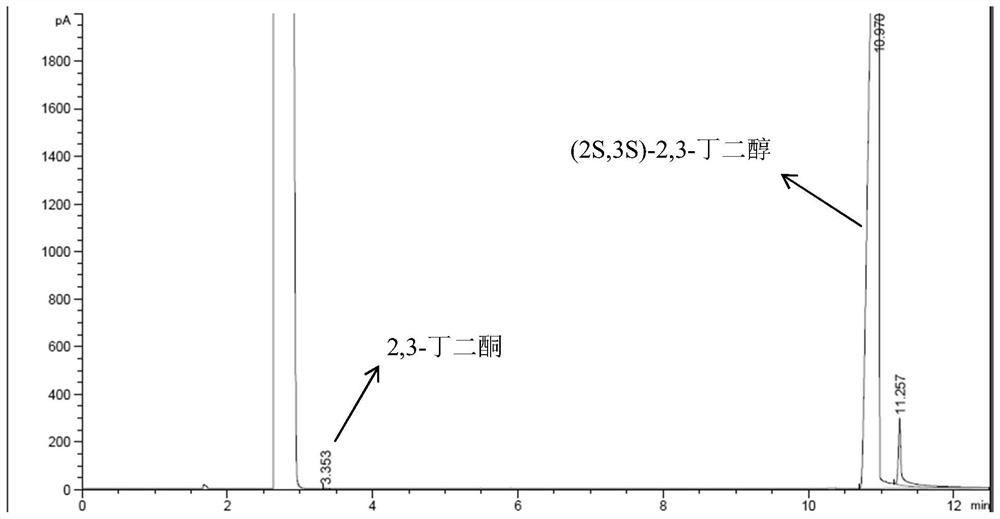
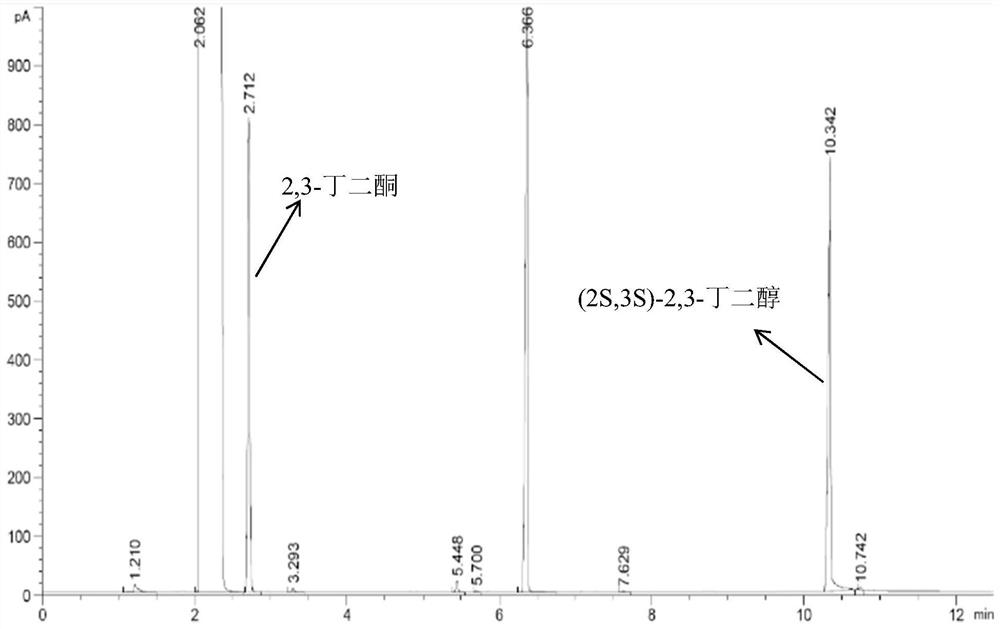
Preparation method of (2S,3S)-2,3-butanediol
A technology of butanediol and diacetyl, which is applied in the field of biochemistry, can solve the problems of low concentration and optical purity, low chiral purity, and low efficiency, and achieve easy-to-obtain raw materials, simple synthetic routes, and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a kind of preparation method that contains the solution of ketoreductase, it comprises the following steps:
[0034] (1) Insert the coding gene of ketoreductase between the Nco I and EcoR I restriction sites of the pET28a (+) carrier to obtain a recombinant vector; the nucleotide sequence of the coding gene of the ketoreductase is shown in the sequence listing Shown in SEQ ID NO: 1; the amino acid sequence of the ketoreductase is shown in SEQ ID NO: 2 in the sequence listing.
[0035] (2) Transform the above recombinant vector into Escherichia coli BL21 to obtain a recombinant strain.
[0036] (3) Inoculate the above-mentioned recombinant strain into LB solid medium containing chloramphenicol, and place it at 37°C for activating culture for 20 hours to obtain colonies; use an inoculation loop to pick a single colony and inoculate it into 50 mL of LB medium (with chloramphenicol added) in a 250mL Erlenmeyer flask, cover the bottle with a sealing...
Embodiment 2
[0039] This embodiment provides a kind of (2S,3S)-2, the preparation method of 3-butanediol, it comprises the following steps:
[0040] (1) Get 6.06g of the solution containing ketoreductase obtained in the above-mentioned embodiment 1 and 20mL concentration of 0.01mol / L phosphate buffered saline solution and place it in a clean 250mL single-necked flask with a magnet, and place the single-necked flask In a water bath at 20° C., the solution in the one-necked flask was stirred and mixed at a speed of 400 rpm to obtain a mixed solution.
[0041] (2) During the stirring process, keep the temperature of the water bath constant, and sequentially add 0.202g of nicotinamide adenine dinucleotide, 100mL of isopropanol and 20.2g of 2,3-butanedione to the above mixture A sealed reaction was carried out to obtain a reaction solution.
[0042] (3) The above reaction solution was filtered under reduced pressure to obtain a light yellow filtrate, and then the filtrate was subjected to rota...
Embodiment 3
[0045] This embodiment provides a kind of (2S,3S)-2, the preparation method of 3-butanediol, it comprises the following steps:
[0046] (1) Get 10.1g of the solution containing ketoreductase obtained in the above-mentioned embodiment 1 and 20mL concentration of 0.2mol / L phosphate buffered saline solution and place it in a clean 250mL single-necked flask with magnets, and place the single-necked flask In a water bath at 45° C., the solution in the one-necked flask was stirred and mixed at a speed of 400 rpm to obtain a mixed solution.
[0047] (2) During the stirring process, keep the temperature of the water bath constant, and sequentially add 0.606g of nicotinamide adenine dinucleotide, 180mL of isopropanol and 20.2g of 2,3-butanedione to the above mixture A sealed reaction was carried out to obtain a reaction solution.
[0048] (3) Suction filtration of the above reaction solution under reduced pressure to obtain a light yellow filtrate, and then place the filtrate at 55° C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com