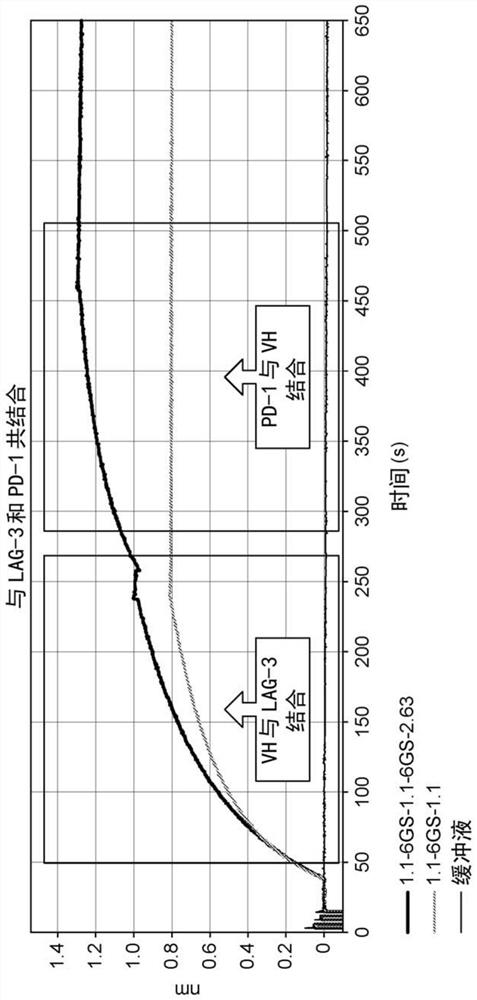
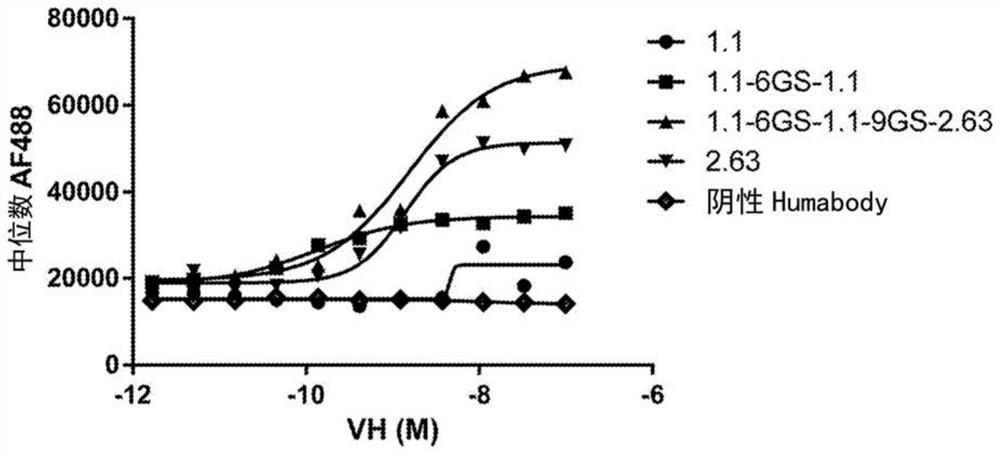
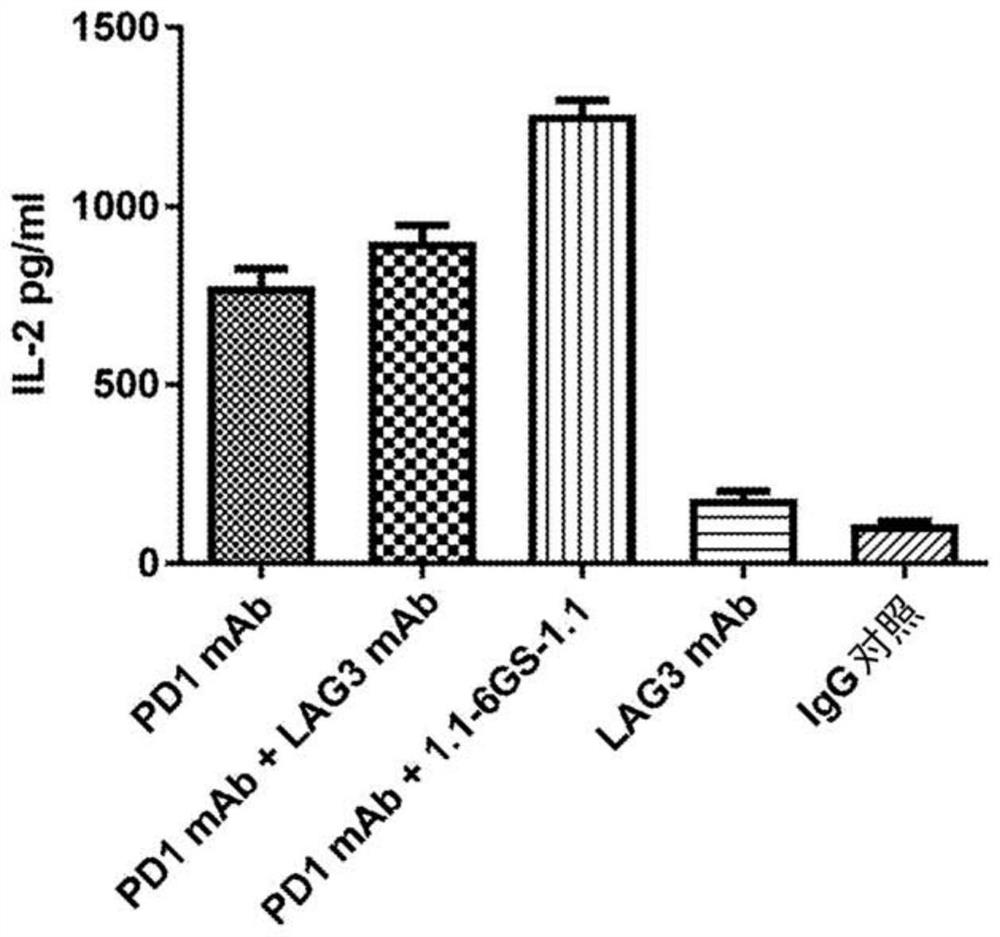
Therapeutic molecules that bind to lag3 and pd1
A technology of LAG-3 and PD-1, applied in the field of protein, can solve problems such as blocking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0366] The construction of embodiment 1Tg / TKO mouse
[0367] Mice carrying the human heavy chain antibody transgenic locus in germline configuration in the context of silencing endogenous heavy and light chain antibody expression (triple knockout, or TKO) were created as previously described (WO2004 / 076618, WO2003 / 000737, Ren et al., Genomics, 84, 686, 2004; Zou et al., J. Immunol., 170, 1354, 2003 and WO2016 / 062990). Briefly, transgenic mice were obtained after pronuclear microinjection of freshly fertilized oocytes with yeast artificial chromosomes (YACs) containing and lacking C H 1 domain of mouse immunoglobulin constant region genes, mouse enhancers and regulatory regions combined with excess human VH, D and J genes. Yeast artificial chromosomes (YACs) are vectors that can be used to clone very large DNA inserts in yeast. They accept large DNA insertions in addition to containing all three cis-acting structural elements (autonomously replicating sequence (ARS), centrom...
Embodiment 2
[0370] Example 2 Antigens used for immunization and characterization
[0371] LAG-3
[0372] Human and monkey LAG-3 extracellular domains were made as IgG fusion proteins for immunization of transgenic mice or for binding studies. Human LAG-3 sequence accession number NM_002286. Rhesus monkey LAG-3 accession number XM_001108923. For LAG3 domains 1-3 (NC_022282), the amino acid sequence of the rhesus monkey matched that of the cynomolgus monkey. Mouse LAG3 was from R&DBiotechne (catalogue 3328-L3). Human LAG-3 domains 1-4 and 1-2 were cloned using standard molecular biology techniques. Domains were identified according to Huard et al. 1997 PNAS 94:5744-9. Expression was performed in HEK 293 cells, and LAG3 protein was purified using size exclusion chromatography. Chinese hamster ovary (CHO) cells expressing human LAG-3 were generated using the human LAG-3 sequence in an inducible expression vector. Plasmids were transfected using lipofection and stable cell pools were se...
Embodiment 4
[0378] Example 4 Generates an anti-LAG3 library from immunized mice
[0379] Generation of libraries from the above immunized mice followed the standard protocol for library generation as described below:
[0380] a) Tissue collection and homogenization, RNA extraction and RT-PCR
[0381] Spleen, inguinal and brachial lymph nodes were collected from several immunized mice to middle. Using Qiagen Kit (cat 74104) RNA was extracted from total spleen using the Pure RNA Tissue kit (cat 98040496) following the manufacturer's protocol or via the KingRNAer automated system (Thermo). V was mined from RNA samples using the Superscript III RT-PCR High-Fidelity Kit (Invitroge cat 12574-035) and according to the manufacturer's protocol. H sequence.
[0382] b) Cloning into a phagemid vector
[0383] The phagemid vector pUCG3 was used in these studies. Conventional PCR-based methods are used to amplify V from H Sequence Construction V H Phagemid library. Purified V H RT-PC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com