Bio-orthogonal activated time-resolved response type rare earth probe and its preparation method and application
A time-resolved, bio-orthogonal technology, applied in the direction of material excitation analysis, chemical instruments and methods, preparations for in vivo experiments, etc., can solve the problems of high copper ion toxicity, unfavorable large-scale promotion, large fluorescence background, etc., to achieve good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
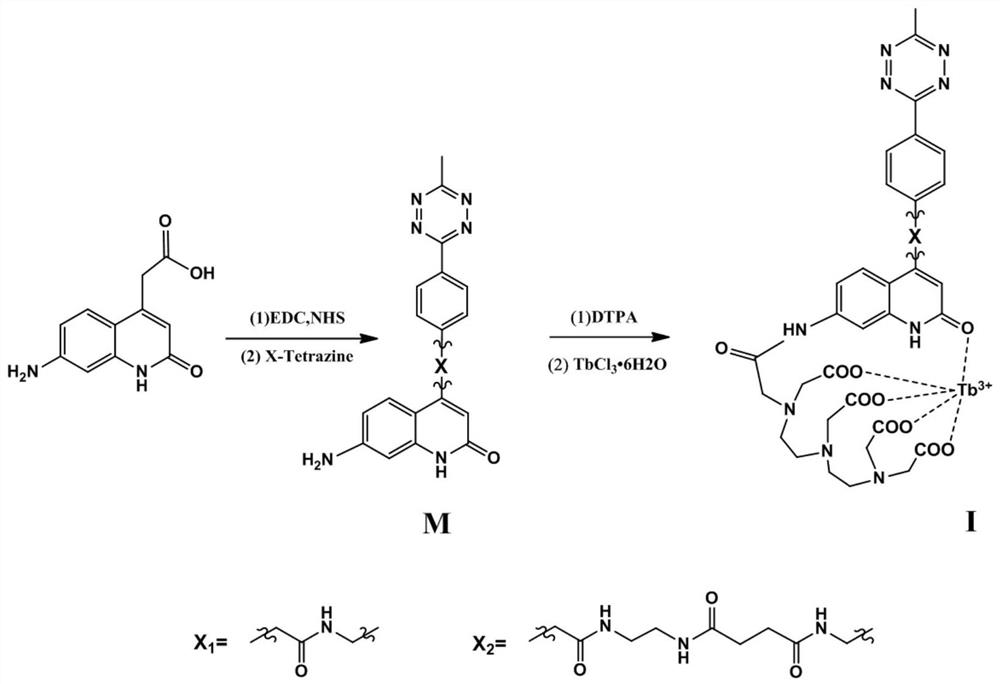
[0044] (1) The synthesis steps of the bio-orthogonal activated time-resolved response type rare earth probes provided by the present invention are as follows: figure 1 As shown, the details are as follows: IX 1 Intermediate S 1 Preparation: 7-amino-2-keto-4-quinolinic acid (43.6mg, 0.2mmol) was mixed with N-hydroxysuccinimide NHS (115.0mg, 1.0mmol), 1-(3-dimethyl Aminopropyl)-3-ethylcarbodiimide hydrochloride EDC·HCl (190.8mg, 1.0mmol) was dissolved in 10mL N,N-dimethylformamide and stirred at room temperature overnight for carboxyl activation. Precipitate with saturated saline, centrifuge and dry to obtain a tan solid (i.e. activated 7-amino-2-keto-4-quinolinic acid); continue to mix the tan solid with 4-(6-methyl-1,2, 4,5-tetrazin-3-yl)benzylamine (40.2mg, 0.2mmol) was stirred in N,N-dimethylformamide at room temperature overnight, the solvent was removed, and the red solid S was obtained by silica gel chromatography. 1 (66.8 mg), yield 83.3%.
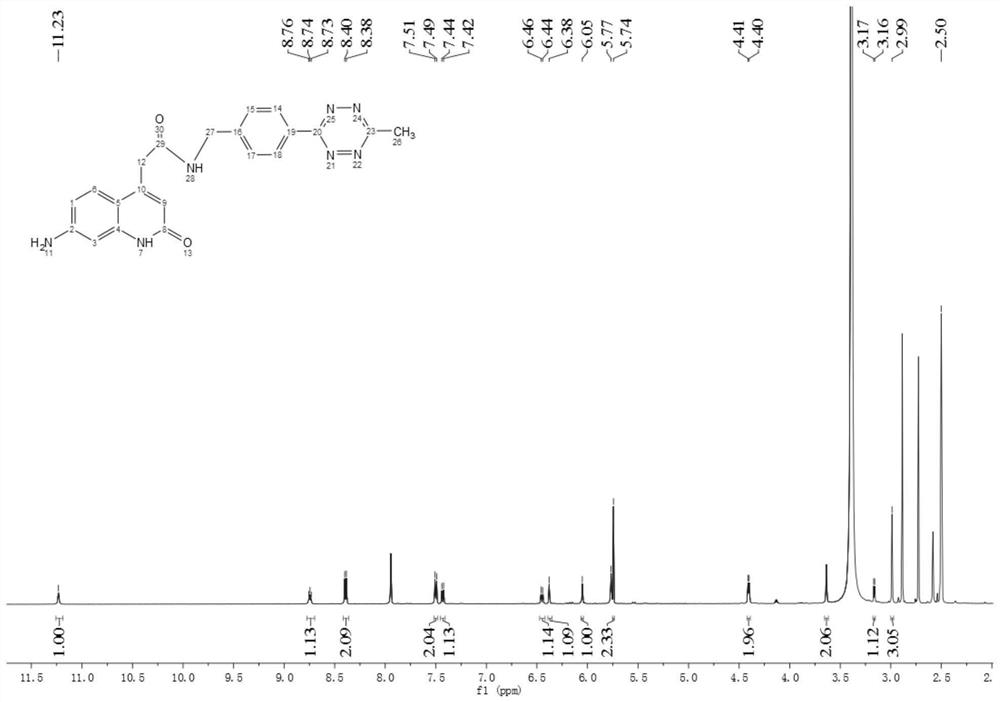
[0045] characterizing dat...
Embodiment 2
[0049] (1) IX 1 Intermediate S 2 Preparation: at room temperature, remove the red solid S obtained in Example 1 1 (20.5mg, 0.05mmol), diethyltriaminepentaacetic acid DTPA (19.7mg, 0.05mmol) were dissolved in 3mL dimethyl sulfoxide, stirred for overnight reaction, and then precipitated with ether and dried to obtain solid S 2 (34.3 mg), yield 88.4%.
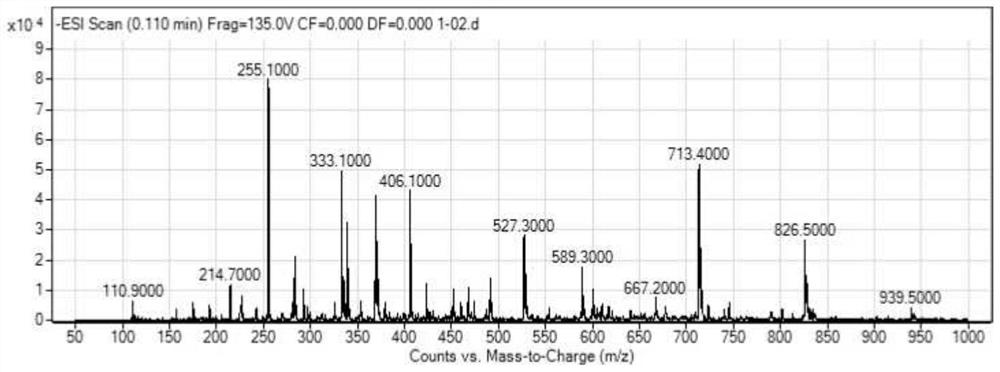
[0050] Characterization data: ICP-MS ( Figure 5 ):calcd.for[M + ]776.76,found:713.40.
[0051] (2) IX 1 Preparation: at room temperature, remove the solid S obtained in step (1) 2 (7.8mg, 0.01mmol), terbium chloride hexahydrate TbCl 3 ·6H 2 O (3.8 mg, 0.01 mmol) was dissolved in 1 mL dimethyl sulfoxide / water (volume ratio, 1:1), stirred overnight for reaction, and then precipitated and dried with ether to obtain rare earth probe IX 1 (8.41 mg), yield 90.1%.
[0052] Characterization data: ICP-MS ( Figure 6 ):calcd.for[M + ]931.66, found: 931.20.
Embodiment 3
[0054] (1) IX 2 Intermediate S 3 , S 4 Preparation: Dissolve the activated 7-amino-2-keto-4-quinolinic acid (33.2 mg, 0.1 mmol) in Example 1 in 10 mL of N,N-diamine containing ethylenediamine (6 mg, 0.1 mmol) In methylformamide, stirred overnight at room temperature, after the completion of the reaction was monitored by TCL, 4-(6-methyl-1,2,4,5-tetrazin-3-yl)benzylamine (30.1 mg, 0.1 mmol) with succinic anhydride (10.0mg, 0.1mmol) was stirred overnight at room temperature in N,N-dimethylformamide, the solvent was removed, and the red solid S was obtained by silica gel chromatography. 3 . At room temperature, the red solid S 3 , diethyltriaminepentaacetic acid (39.5mg, 0.1mmol) was dissolved in 4mL dimethyl sulfoxide, stirred for overnight reaction, then precipitated and dried with ether to obtain its solid S 4 (68.3 mg), yield 74.4%.
[0055] S 3 Characterization data: ICP-MS ( Figure 7 ):calcd.for[M + ]526.59,found:525.10.
[0056] red solid S 3 The structural for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com