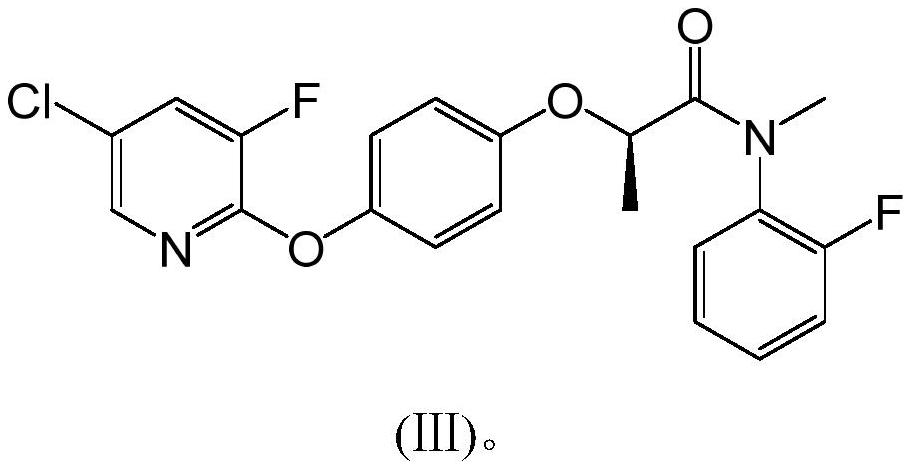
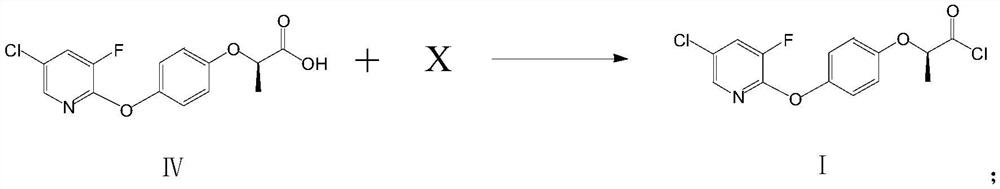Pyridyloxy phenoxy propionamide compound with herbicidal activity and synthesis method and application thereof
A synthesis method and compound technology, applied in herbicides and algicides, botanical equipment and methods, organic chemistry, etc., can solve problems such as production and storage restrictions, and achieve good control performance and the effect of small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 322g of toluene and 115.8g of compound IV into a 1000ml three-necked flask, stir at a temperature of 60-65°C for 30min, add 119g of thionyl chloride dropwise within about 1 hour, and after the addition is complete, raise the temperature to 80°C and keep it for 2h to obtain The reaction solution of compound I.
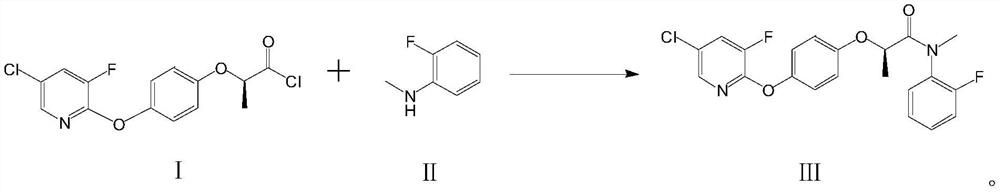
[0045]Add 62.5g of compound II, 230g of toluene, and 120g of sodium hydroxide into a 2000ml three-neck flask, stir; keep the temperature at 20-25°C, and add the reaction solution containing compound I dropwise to the mixed solution containing compound II within about 1.5 hours After the dropwise addition, keep warm at 20-25°C for 2h. After the reaction is over, filter to remove the inorganic salts and excessive inorganic bases generated by the reaction, add 10% hydrochloric acid dropwise to the filtrate to adjust the pH value to 2, separate layers, rotate the organic phase, add 300g of 90% ethanol, crystallize at 0-5 ° C, filter, After drying, 196 g of compo...
Embodiment 2
[0049] Add 322g of toluene and 115.8g of compound Ⅳ into a 1000ml three-neck flask, stir at a temperature of 60-65°C for 30min, add 89.2g of thionyl chloride dropwise within about 1 hour, after the addition is complete, raise the temperature to 80°C and keep it for 2h, and set aside .
[0050] Add 62.5g of compound II, 230g of toluene, and 120g of sodium hydroxide into a 2000ml three-neck flask, stir, keep the temperature at 20-25°C, and add the reaction solution containing compound I dropwise to the mixed solution containing compound II within about 1.5 hours After the dropwise addition, keep warm for 2h. After the reaction is completed, filter, add 10% hydrochloric acid dropwise to the filtrate to adjust the pH value to 2, separate layers, rotate the organic phase, add 300g 90% ethanol, crystallize at 0-5°C, filter, and dry to obtain 202g of finished product with a content of 98.2%. The rate is 96.46%.
Embodiment 3
[0052] Add 322g of toluene and 115.8g of compound Ⅳ into a 1000ml three-neck flask, stir at a temperature of 60-65°C for 30min, add 89.2g of thionyl chloride dropwise within about 1 hour, after the addition is complete, raise the temperature to 80°C and keep it for 2h, and set aside .
[0053] Add 62.5g of compound II, 230g of toluene, and 168g of potassium hydroxide into a 2000ml three-necked flask, stir, keep the temperature at 20-25°C, and add the reaction solution containing compound I dropwise to the mixed solution containing compound II within about 1.5 hours After the dropwise addition, keep warm for 2h. After the reaction is completed, filter, add 10% hydrochloric acid dropwise to the filtrate to adjust the pH value to 2, separate layers, rotate the organic phase, add 300 g of 90% ethanol, crystallize at 0-5 ° C, filter, and dry to obtain 194 g of finished product with a content of 97.4%. The rate is 92.64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com