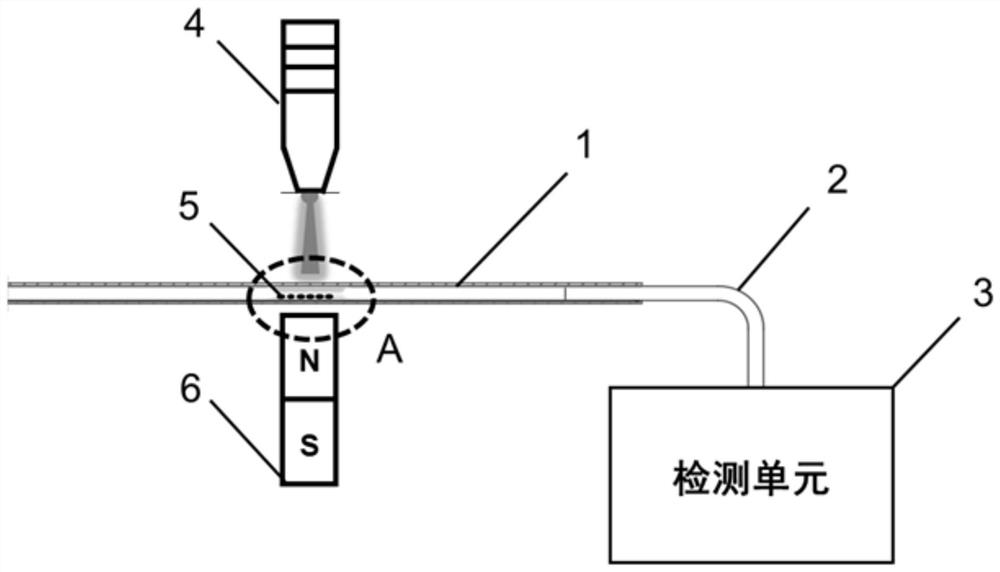
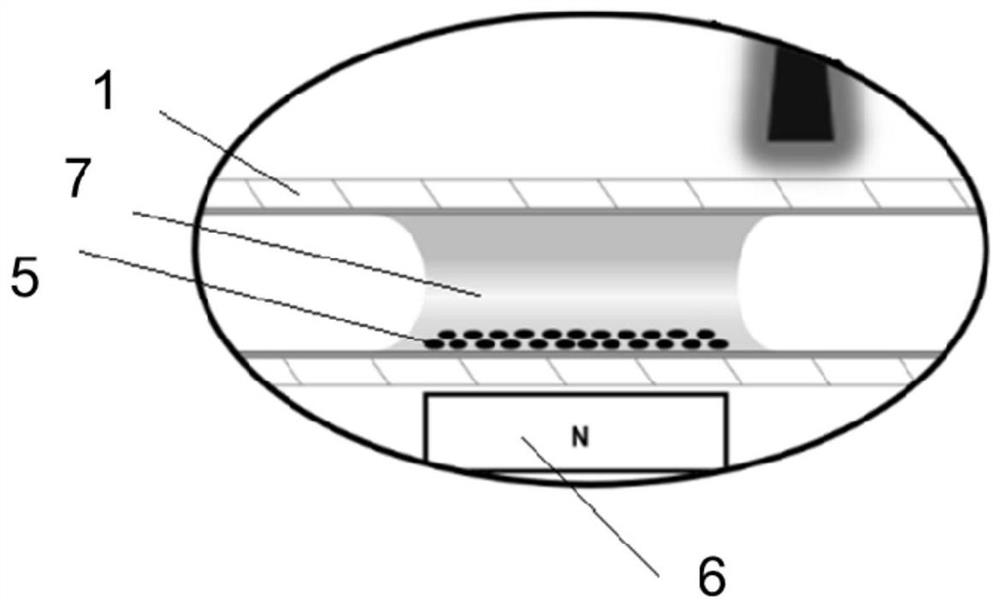
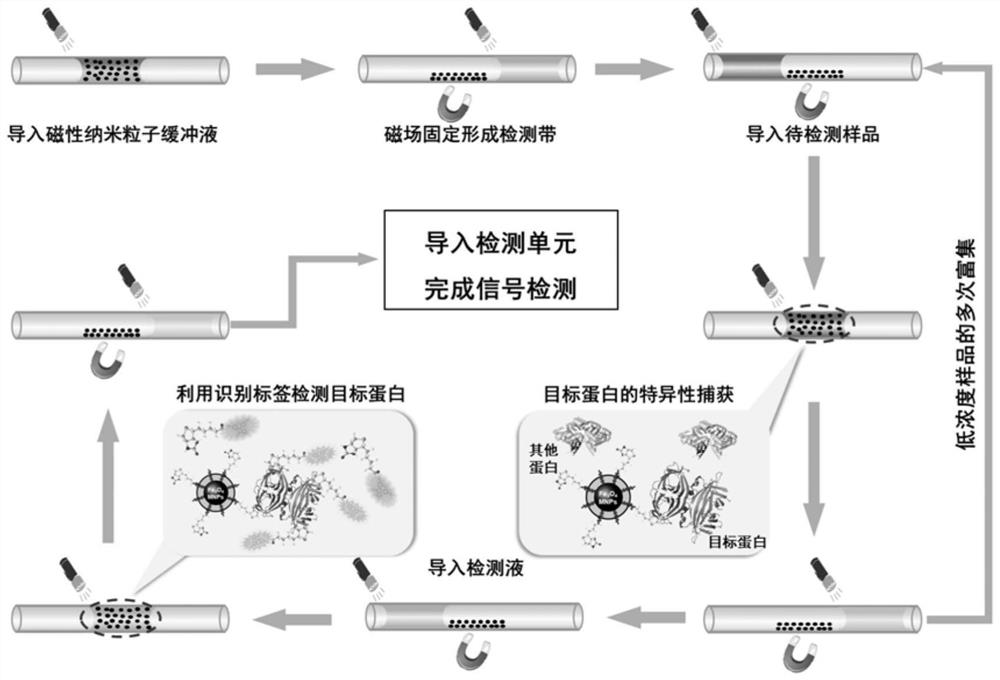
Protein enrichment detection device based on light-operated fluid transportation and magnetic control sample separation
A detection device and protein technology, applied in the field of microfluidics, can solve problems such as hindering the miniaturization development of microfluidic devices, low reaction efficiency, waste of samples, etc., and achieve portable protein detection, increase control accuracy, and reduce volume. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Single enrichment and detection of high concentration Avidin protein
[0104] 1. Inject 5 μL of magnetic nanoparticle solution of surface-modified Biotin molecules at a concentration of 0.5 mg / mL into the optofluidic microtube actuator 1. After the optically controlled particle solution moves to the designated position, a magnetic field is applied to fix the magnetic nanoparticles to form a magnetic field. Nanoparticle capture zone 5. Then the light-controlled supernatant is separated from the magnetic nanoparticle capture zone 5 .
[0105] 2. Inject 5 μL of the Avidin sample to be detected into the optofluidic microtube actuator 1, and after the optically controlled sample to be detected reaches the magnetic nanoparticle capture zone 5, the magnetic field is removed, and the optically controlled solution reciprocates near the magnetic nanoparticle capture zone 5 , resuspend the magnetic nanoparticles, and continue the reciprocating stirring motion for 10 minutes (the ...
Embodiment 2
[0108] Multiple enrichment and detection of low concentration Avidin protein
[0109] 1. Inject 5 μL of magnetic nanoparticle solution of surface-modified Biotin molecules at a concentration of 0.5 mg / mL into the optofluidic microtube actuator 1. After the optically controlled particle solution moves to the designated position, a magnetic field is applied to fix the magnetic nanoparticles to form a magnetic field. Nanoparticle capture zone 5. Then the light-controlled supernatant is separated from the magnetic nanoparticle capture zone 5 .
[0110] 2. Inject 5 μL of the Avidin sample (lower concentration) to be detected into the optofluidic microtube actuator 1. After the light-controlled sample to be detected reaches the magnetic nanoparticle capture zone 5, the magnetic field is removed, and the light-controlled solution is captured by the magnetic nanoparticles. Reciprocate around belt 5 to resuspend the magnetic nanoparticles, and continue to reciprocate and stir for 10 m...
Embodiment 3
[0114] Multiple enrichment and detection of C-reactive protein
[0115] 1. Introduce particles and fix the capture band: Introduce 5 μL of the magnetic nanoparticle solution of the surface-modified C-reactive protein primary antibody with a concentration of 0.5 mg / mL into the optofluidic microtube actuator 1, move to the designated position, and apply a magnetic field to fix it. Live MNPs to form magnetic nanoparticle trapping strips 5 . The light control particle supernatant is separated from the magnetic nanoparticle capture zone 5 .
[0116] 2. Capture and separation of C-reactive protein samples: import 5 μL of C-reactive protein samples to be detected, light-control the samples to be detected to reach the particle capture zone, remove the magnetic field, light-control the reciprocating motion of the solution, resuspend MNPs, and continue to reciprocate 10min (the reciprocating cycle is 10s), during this process, the C-reactive protein primary antibody on the surface of M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com