Pure cobalt hydrotalcite compound and preparation method thereof
A hydrotalcite compound, hydrothermal aging technology, applied in cobalt compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of cobalt ion residues and underutilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
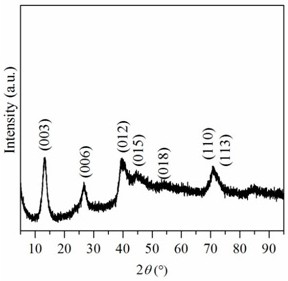
[0040] A preparation method of pure cobalt-like hydrotalcite compound, comprising the steps of: weighing 11.1895 g Co(NO 3 ) 2 ·6H 2 O was dissolved in 256mL methanol-water mixed solvent, and the volume ratio of methanol and water was 1:1 to obtain cobalt nitrate solution. Weigh 0.5096g anhydrous Na 2 CO 3 Dissolve in 464mL methanol-water mixed solvent to get Na 2 CO 3 solution. Weigh 6.4000g NaOH and dissolve in 80mL methanol-water mixed solvent to obtain NaOH solution. Under room temperature and vigorous stirring, the cobalt nitrate solution was slowly dropped into Na 2 CO 3 solution, while adjusting the reaction system pH=10 with NaOH solution. After the cobalt nitrate solution was added dropwise, the obtained precipitate was filtered and washed with a methanol-water mixed solution, and the obtained solid was dried in an oven at 60 °C for 12 hours to obtain a pure cobalt-like hydrotalcite compound.
[0041] The crystal phase structure of the above samples was char...
Embodiment 2
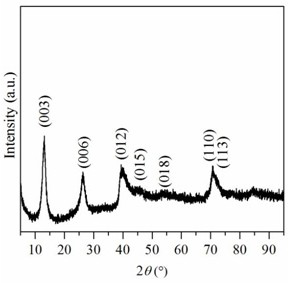
[0043] A preparation method of pure cobalt-like hydrotalcite compound, comprising the steps of: weighing 11.1895g Co(NO 3 ) 2 ·6H 2 O was dissolved in 256mL methanol-water mixed solvent, the volume ratio of methanol and water was 4:1 to obtain cobalt nitrate solution. Weigh 0.5096 g anhydrous Na 2 CO 3 Dissolved in 464 mL of methanol-water mixed solvent to obtain Na 2 CO 3 solution. Weigh 6.4000 g NaOH and dissolve it in 80 mL methanol-water mixed solvent to obtain NaOH solution. Under room temperature and vigorous stirring, the cobalt nitrate solution was slowly dropped into Na 2 CO 3 solution, while adjusting the reaction system pH=10 with NaOH solution. After the cobalt nitrate solution was added dropwise, the obtained precipitate was filtered and washed with a methanol-water mixed solution, and the obtained solid was dried in an oven at 60 °C for 12 hours to obtain a pure cobalt-like hydrotalcite compound.
[0044] The crystal phase structure of the above samples...
Embodiment 3
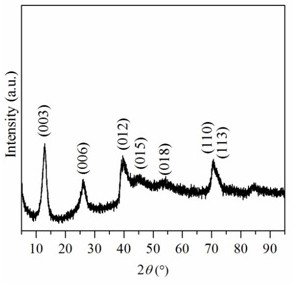
[0046] A preparation method of pure cobalt-like hydrotalcite compound, comprising the steps of: weighing 11.1895g Co(NO 3 ) 2 ·6H 2 O was dissolved in 256mL methanol-water mixed solvent, the volume ratio of methanol and water was 8:1 to obtain cobalt nitrate solution. Weigh 0.5096 g anhydrous Na 2 CO 3 Dissolved in 464 mL of methanol-water mixed solvent to obtain Na 2 CO 3 solution. Weigh 6.4000 g NaOH and dissolve it in 80 mL methanol-water mixed solvent to obtain NaOH solution. Under room temperature and vigorous stirring, the cobalt nitrate solution was slowly dropped into Na 2 CO 3 solution, while adjusting the reaction system pH=10 with NaOH solution. After the cobalt nitrate solution was added dropwise, the obtained precipitate was filtered and washed with a methanol-water mixed solution, and the obtained solid was dried in an oven at 60 °C for 12 hours to obtain a pure cobalt-like hydrotalcite compound.
[0047] The crystal phase structure of the above samples...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com