L-glutamic acid derivative and synthesis method and application thereof
A synthesis method and technology of glutamic acid are applied in the field of synthesis of intermediates of medicine and traditional Chinese medicine, and can solve the problems of complicated production process, inability to apply in the field of peptide synthesis, and high energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
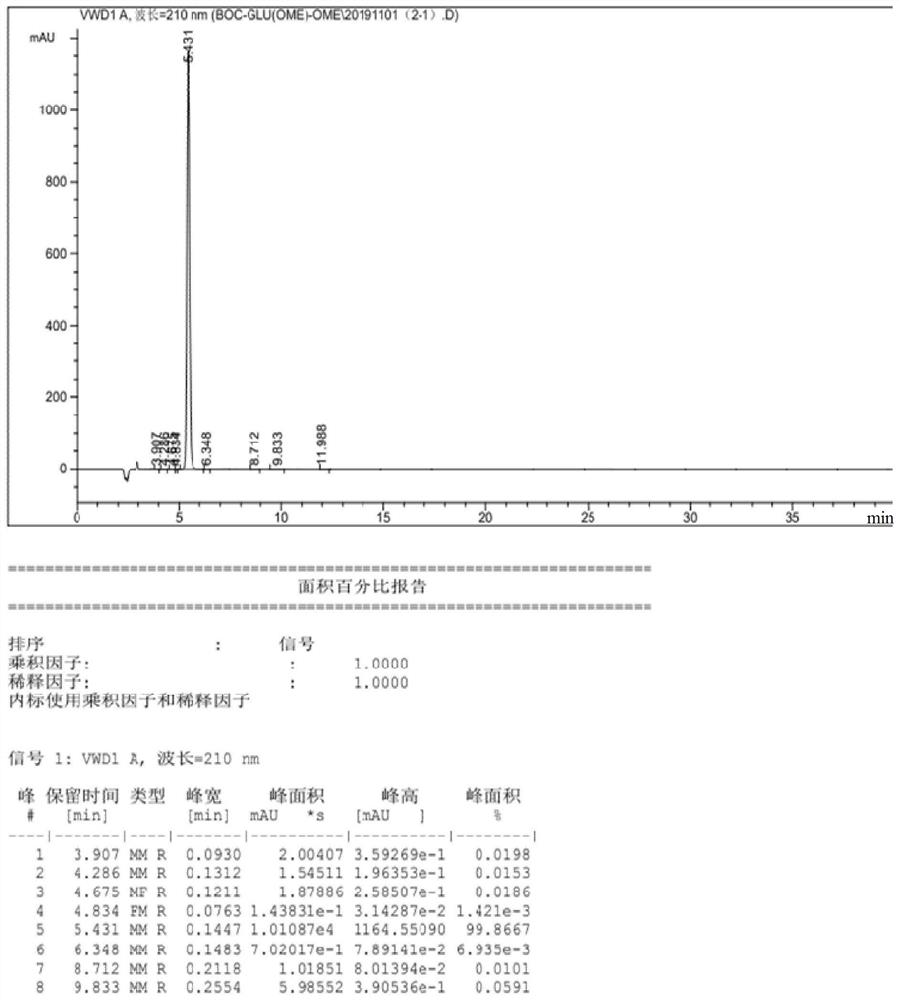
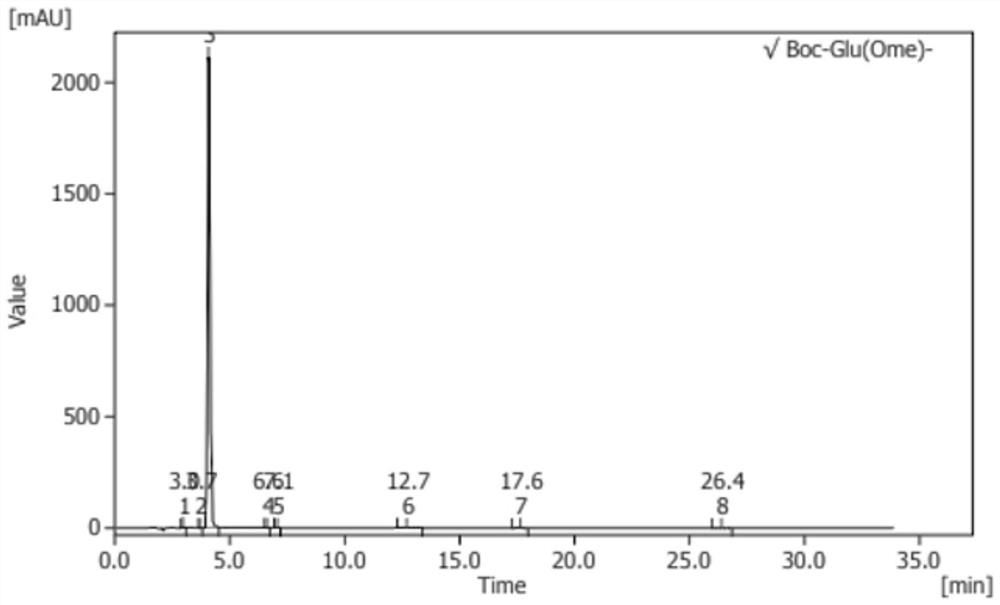
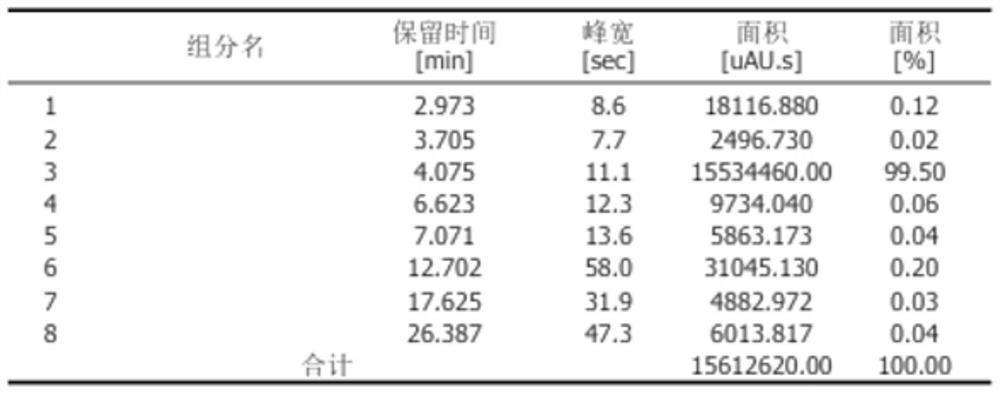
Image
Examples
Embodiment 1
[0045] A kind of synthetic method of L-glutamic acid derivative, synthetic method comprises the steps:
[0046](1) Select 300g (2.04mol) of L-glutamic acid (L-Glu-OH) and 1kg (1.25L, 31mol) of methanol (MeOH) into the reaction device for mixing, and add chlorine at 8-10 degrees Celsius Sulfoxide (SOCl 2 ) 250g (2.1mol), then heat the temperature to 30-35 degrees Celsius and keep the constant temperature reaction for 3 hours to obtain the standby solution I;
[0047] (2) Concentrate the stock solution I after the complete reaction to dryness at 40-45 degrees Celsius, and cool to room temperature to obtain L-glutamic acid dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl );
[0048] (3) Add ethyl acetate 1kg (1.1L, 11.36mol) to the L-glutamate dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl) obtained in step (2), Water 200ml, adjust the pH to 9-10 with sodium hydroxide, then add (Boc) 2 O (di-tert-butyl dicarbonate) 440g (2.02mol) was reacted for 3 hours to obtain standby soluti...
Embodiment 2
[0057] A kind of synthetic method of L-glutamic acid derivative, synthetic method comprises the steps:
[0058] (1) Select 300g (2.04mol) of L-glutamic acid (L-Glu-OH) and 1kg (1.25L, 31mol) of methanol (MeOH) into the reaction device for mixing, and add chlorine at 8-10 degrees Celsius Sulfoxide (SOCl 2 ) 250g (2.1mol), then heat the temperature to 30-35 degrees Celsius and keep the constant temperature reaction for 3 hours to obtain the standby solution I;
[0059] (2) Concentrate the stock solution I after the complete reaction to dryness at 40-45 degrees Celsius, and cool to room temperature to obtain L-glutamic acid dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl );
[0060] (3) Add ethyl acetate 1kg (1.1L, 11.36mol) to the L-glutamate dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl) obtained in step (2), 200ml of water, adjust the pH to 9-10 with sodium hydroxide, then add 440g (2.02mol) of di-tert-butyl dicarbonate and react for 3 hours to obtain the standby solution I...
Embodiment 3
[0067] A kind of synthetic method of L-glutamic acid derivative, synthetic method comprises the steps:
[0068] (1) Select 500g (3.4mol) of L-glutamic acid (L-Glu-OH) and 1.7kg (2.2L, 53mol) of methanol (MeOH) and put them into the reaction device for mixing, and add them at 10-12 degrees Celsius Thionyl chloride (SOCl 2 ) 425g (3.6mol), then heated the temperature to 38-40 degrees Celsius and kept the constant temperature reaction for 12 hours to obtain the standby solution I;
[0069] (2) Concentrate the stock solution I after the complete reaction to dryness at 70-80 degrees Celsius and cool to room temperature to obtain L-glutamic acid dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl );
[0070] (3) Add ethyl acetate 1.7Kg (1.9L, 19mol) to the L-glutamate dimethyl hydrochloride oil (L-Glu(ome)-ome.HCl) obtained in step (2), 350ml of water, adjust the pH to 9-10 with sodium hydroxide, then add 740g (3.4mol) of di-tert-butyl dicarbonate and react for 12 hours to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com