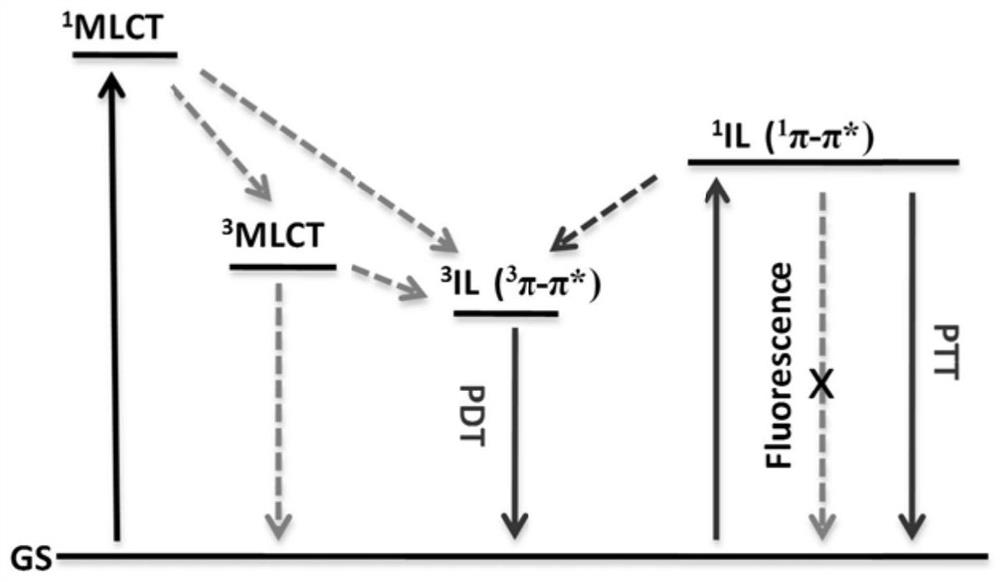
D-A-D structure-based metal complexes with near-infrared light absorption function, and application thereof
A technology of metal complexes and complexes, applied in the direction of ruthenium organic compounds, indium organic compounds, platinum group organic compounds, etc., can solve the problems of short absorption wavelength, hindering the clinical application of metal photosensitizers, and weak skin penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of complex 1:
[0053]
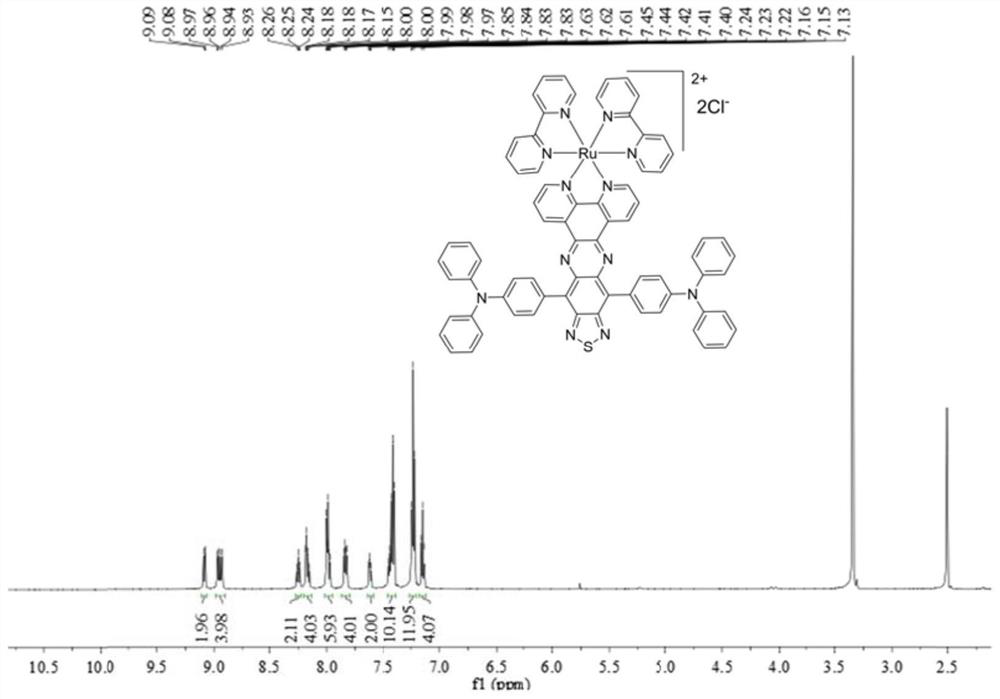
[0054] Cis-(bpy) 2 RuCl 2 (48.40mg, 0.10mmol), ligand L (82.6mg, 0.1mmol) in EtOH / H 2 Mix in O (30 / 10ml), stir and reflux at 80°C for 12h to obtain a dark green solution, remove the solvent, and perform column chromatography with DCM:MeOH=10:1 to obtain a green powder. Yield: 45.5%. Anal. Calcd(%) for C 74 h 50 Cl 2 N 12 RuS: C 67.78, H 3.84, N 12.82. Found: C 67.63, H 3.82, N 12.85; 1 HNMR (600MHz, DMSO-d 6 )δ7.13-7.16(t, 4H, J=7.4Hz), 7.22-7.24(m, 12H), 7.40-7.45(m, 10H), 7.61-7.63(t, 2H, J=6.6Hz), 7.82 -7.85(m,4H),7.97-8.00(m,6H),8.15-8.18(m,4H),8.24-8.26(t,2H,J=7.8Hz),8.93-8.97(m,4H),9.08 -9.09 (m, 2H, J = 7.8 Hz) ppm. NMR spectrum such as figure 2 shown.
Embodiment 2
[0056] Synthesis of complex 2:
[0057]
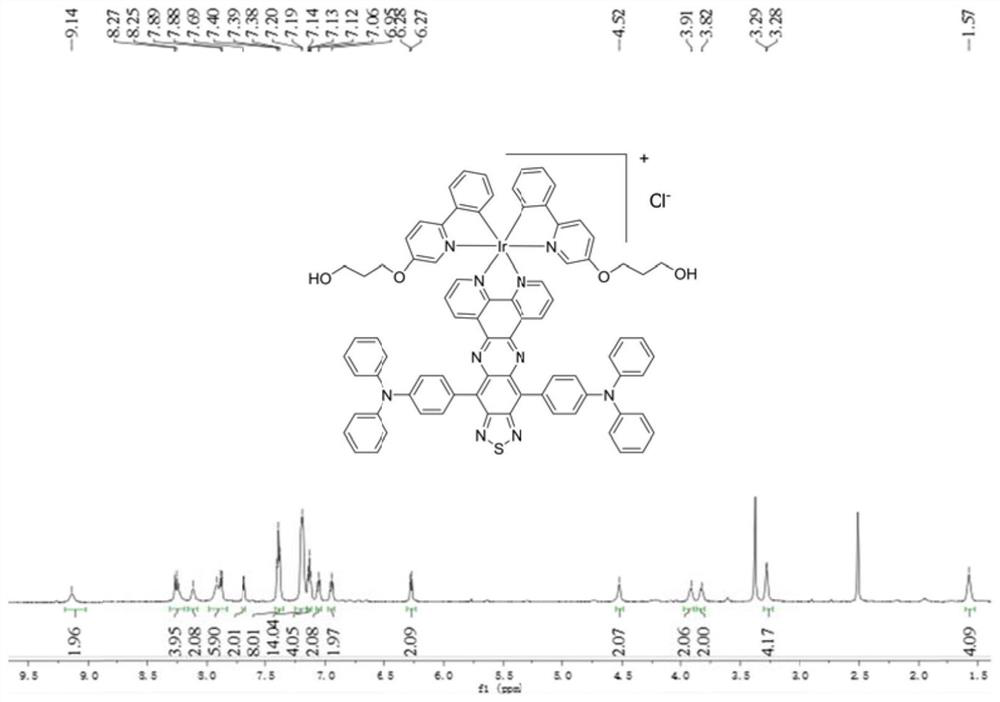
[0058] Dimer of iridium (1.0mmol), ligand L (2.0mmol) in CH 2 Cl 2 / MeOH (20 / 20ml), stirred and refluxed at 40°C for 24h, the solvent was removed, column chromatography DCM:MeOH=10:1 gave a bright green powder. Yield: 45.7%. Bright-green powder. Anal. Calcd (%) for C 82 h 62 ikB 10 o 4 S: C65.17, H 4.14, N 9.27. Found: C65.04, H 4.16, N 9.21; 1 H NMR (600MHz, DMSO-d 6 )δ1.57(m,4H),3.28(m,4H),3.82(m,2H),3.91(m,2H),4.52(m,2H),6.27-6.28(d,2H,J=7.3Hz ), 6.94-6.96(t, 2H, J=7.3Hz), 7.05-7.07(t, 2H, J=7.5Hz), 7.12-7.14(t, 4H, J=7.2Hz), 7.19-7.20(m, 14H), 7.38-7.40(m, 8H), 7.68-7.69(d, 2H, J=7.9Hz), 7.88-7.92(m, 6H), 8.12(m, 2H), 8.24-8.27(m, 4H) ,9.14(m,2H)ppm. NMR spectrum such as image 3 shown.
Embodiment 3
[0060] Synthesis of complex 3:
[0061]
[0062] dimer of aromatic ruthenium (1.0mmol), ligand L (2.0mmol) in CH 2 Cl 2 / MeOH (40 / 20ml), stirred and refluxed at 40°C for 24h, the solvent was removed, and column chromatography DCM:MeOH=15:1 gave a green powder with a yield of 43.7%. 1 H NMR (600MHz, DMSO-d 6 )δ0.98-0.99(d,6H,J=6.2Hz),2.25(m,3H),2.69(m,1H),6.24(m,2H),6.47(m,2H),7.15-7.20(m ,8H),7.22-7.23(d,8H,J=7.6Hz),7.42-7.45(m,8H),7.79(m,4H),8.15(m,2H),8.98(m,2H),10.04( m,2H)ppm. NMR spectrum such as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com