COVID-19 nucleic acid releasing agent and nucleic acid detection kit
A technology of COVID-19 and nucleic acid release agent, which is applied in the direction of DNA preparation, recombinant DNA technology, microbial measurement/inspection, etc. It can solve the problems of short detection time, inability to achieve rapid diagnosis, long detection process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A COVID-19 nucleic acid release agent, comprising: a surfactant with a mass volume percent concentration of 0.02-0.05%, and a buffer reagent with a pH value of 7.0-8.0;
[0066] The surfactant is selected from: NP40, X-100 and Tween20.
[0067] Include Tris-HCl, KCl, NaCl, DTT, (NH 4 ) 2 SO 4 and spermidine.
[0068] The concentration of the KCl is 40mM~50mM, the concentration of the NaCl is 5~8mM; the concentration of the DTT is 1~1.5mM; the (NH 4 ) 2 SO 4 The concentration of the spermidine is 5-8mM; the concentration of the spermidine is 1-1.5%.
Embodiment 2
[0070] A COVID-19 nucleic acid detection kit, comprising:
[0071] 1) the nucleic acid releasing agent of embodiment 1;
[0072] 2) Specific primer pairs and specific fluorescent probes for amplifying the virus sequence ORF1 region, N protein sequence, and monitoring the endogenous reference gene RnaseP of the detection process, the working concentration of the above primer pairs and probes is 10 μM ( 10 μmol / L), as shown in the table below.
[0073] Table 1. Specific primer pairs and specific fluorescent probe primer sequences
[0074] Primer / Probe Name Sequence (5'-3') serial number ORF1a-F AATGGTCATGTGTGGCGGTTCAC SEQ NO.1 ORF1a-R CAGCTTGACAAATGTTAAAAACACTA SEQ NO.2 ORFN-F CAGGCAGCAGTAGGGGAACTTCT SEQ NO.4 ORFN-R GGCCTTTACCAGACATTTTGCTCTCA SEQ NO.5 RNaseP-F CTGTGTGTCCACTCAGGCTTGT SEQ NO.7 RNaseP-R ACAGATGGGTCTCAGGTGCAG SEQ NO.8 ORF1a-P CTCATCAGGAGATGC CACAACTGCT SEQ NO.3 ORFN-P CTGCTCTTGCTTTG...
Embodiment 3
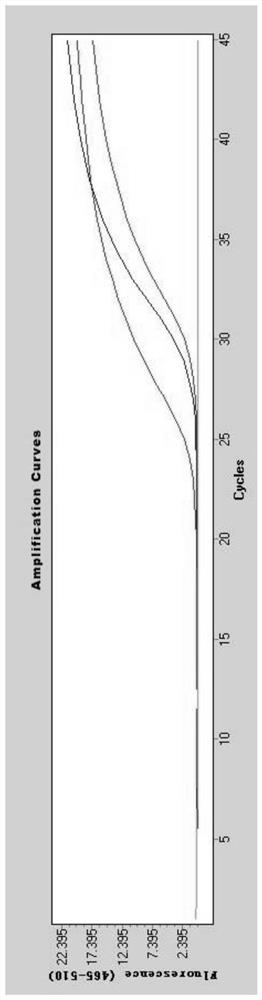
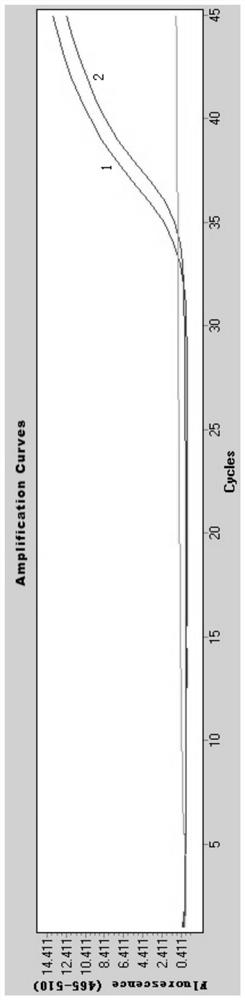
[0078] Adopt the kit of embodiment 2, carry out nucleic acid detection to COVID-19, method flow is as follows:
[0079] 1) Viral nucleic acid release:
[0080] Take 10 μL of a throat swab sample, add 10 μL of nucleic acid release agent, mix well, and let stand at room temperature for 10 minutes.
[0081] 2) RT-PCR system preparation:
[0082] Add the sample (20 μL) processed in step 1) to the RT-PCR reaction system, the RT-PCR reaction is 50 μL, including 5 μL of the primer / probe combination (each primer and probe in Table 1 of Example 3), 2 μL of enzyme solution (reverse transcriptase and Tag enzyme), 23 μL of reaction buffer (remaining components in the RT-PCR reaction reagent in Example 2); the RT-PCR reaction system was dispensed into 96-well plates.
[0083] 3) Put the 96-well plate containing the RT-PCR reaction system prepared in step 2) into the Roche 480 or ABI7500 instrument, set the reaction program, and start real-time detection.
[0084] 4) Result analysis: jud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com