1-((5-tert-butyl-2-hydroxyaniline) methylene)-2-naphthalenone and preparation method and application thereof
A technology of hydroxyaniline and methylene, applied in the field of medicine, can solve problems such as toxic and side effects, and achieve the effects of low production cost, good social and economic benefits, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
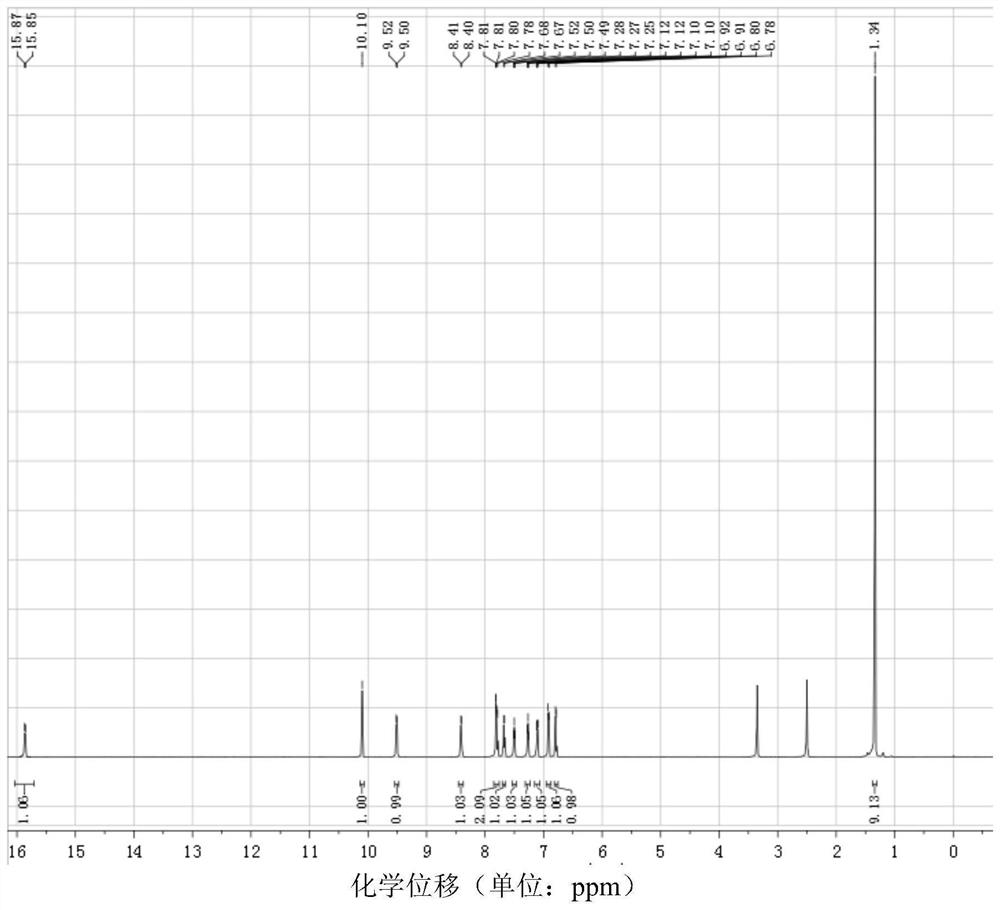
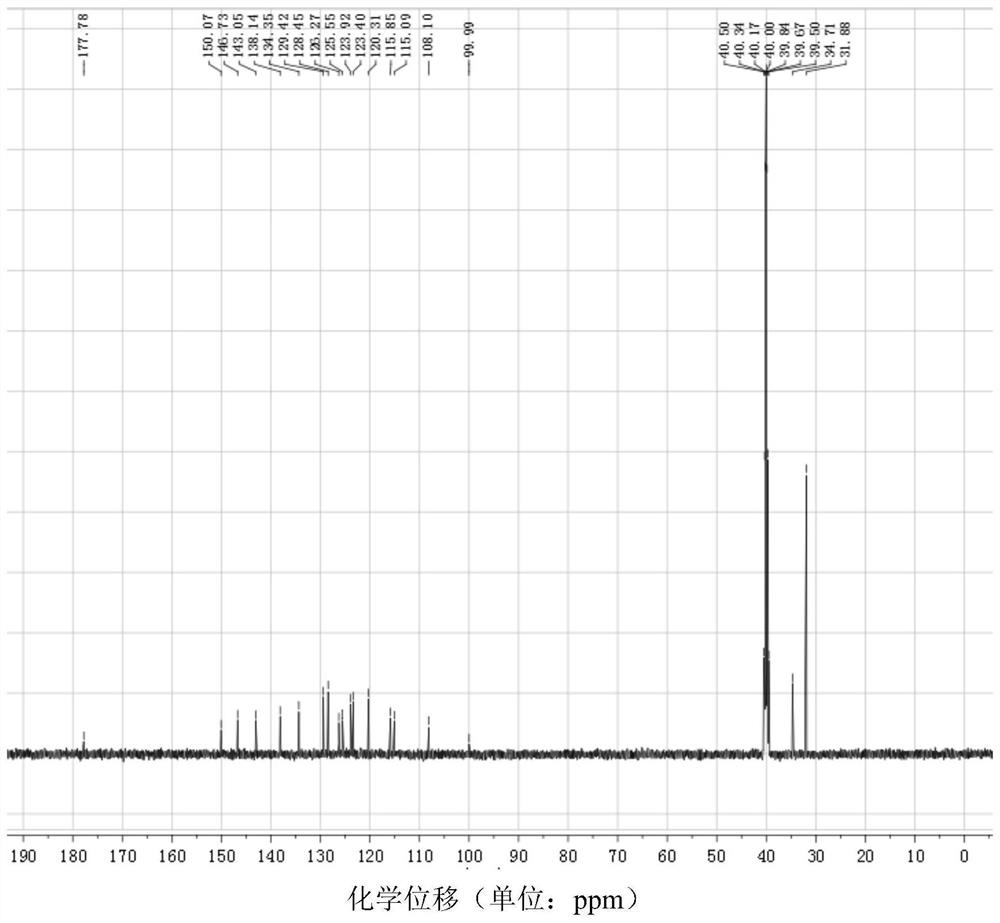
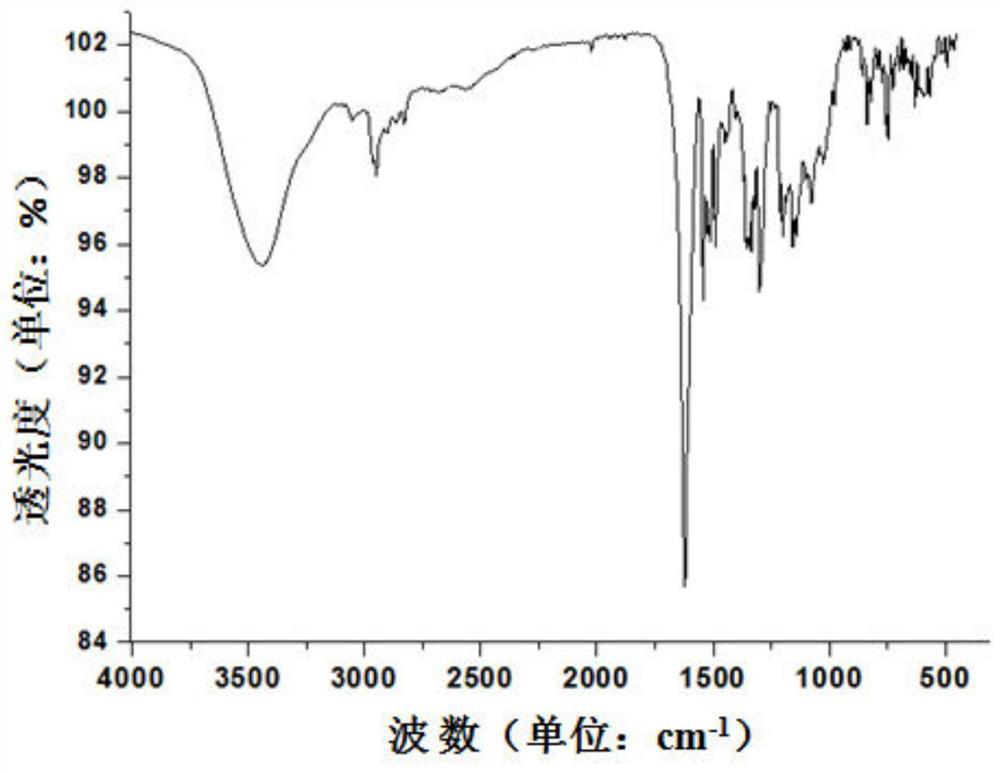
[0032] 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, the structural formula is as follows:
[0033]
[0034] A preparation method of 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, comprising the following steps:
[0035] (1) adding 2-amino-4-tert-butylphenol and 2-hydroxy-1-naphthaldehyde in the reaction flask in a molar ratio of 1:1;
[0036] (2) in the reaction flask of step (1), add dehydrated alcohol, (2-amino-4-tert-butylphenol+2-hydroxy-1-naphthalene carboxaldehyde): the mass ratio of dehydrated alcohol is 1:100, in 80℃ reflux reaction for 8h;
[0037] (3) the reaction solution of step (2) is suction-filtered to obtain solid, fully washed with dehydrated alcohol and dried, and then recrystallized in dehydrated alcohol to obtain brick-red solid powder, which is 1-((5-tertiary Butyl-2-hydroxyaniline)methylene)-2-naphthone crude product;
[0038] (4) Dissolve 0.5mmol of brick red solid powder in 20ml of methanol completely, stir for 1h, filter, ...
Embodiment 2
[0048] 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, the structural formula is as follows:
[0049]
[0050] A preparation method of 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, comprising the following steps:
[0051] (1) adding 2-amino-4-tert-butylphenol and 2-hydroxy-1-naphthalene carboxaldehyde in a molar ratio of 1:1.2 to the reaction flask;
[0052] (2) Methanol was added to the reaction flask in step (1), the mass ratio of (2-amino-4-tert-butylphenol+2-hydroxy-1-naphthalenecarboxaldehyde):methanol was 1:40, and refluxed at 75°C Reaction for 10h;
[0053] (3) the reaction solution of step (2) is suction filtered to obtain solid, fully washed with dehydrated alcohol and dried, and then recrystallized in dehydrated ethanol to obtain brick-red solid powder, which is 1-((5-tertiary Butyl-2-hydroxyaniline)methylene)-2-naphthone crude product;
[0054] (4) Dissolve 0.1 mmol of brick red solid powder in 10 ml of methanol completely, stir for 2 h, ...
Embodiment 3
[0057] 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, the structural formula is as follows:
[0058]
[0059] A preparation method of 1-((5-tert-butyl-2-hydroxyaniline)methylene)-2-naphthone, comprising the following steps:
[0060] (1) adding 2-amino-4-tert-butylphenol and 2-hydroxy-1-naphthaldehyde in a molar ratio of 1.2:1 to the reaction flask;
[0061] (2) Add methanol to the reaction flask of step (1), (2-amino-4-tert-butylphenol+2-hydroxy-1-naphthalenecarboxaldehyde):methanol mass ratio of 1:150, and reflux reaction at 85°C 7h;
[0062] (3) the reaction solution of step (2) is suction filtered to obtain a solid, fully washed with dehydrated alcohol and dried, and then recrystallized in dehydrated alcohol to obtain a brick-red solid powder, which is 1-((5-tertiary Butyl-2-hydroxyaniline)methylene)-2-naphthone crude product;
[0063] (4) Dissolve 0.3mmol of brick red solid powder in 15ml of methanol completely, stir for 1h, filter, and the filtrate is nat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com