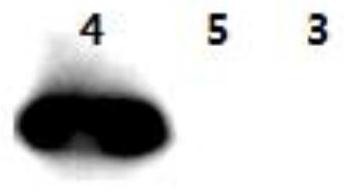
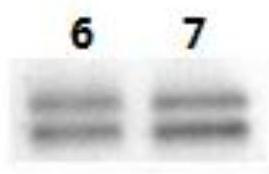
Phosphorylated antigen polypeptide, phosphorylated antibody and preparation method for phosphorylated antigen antibody
An antigen peptide, phosphorylation technology, applied in biochemical equipment and methods, chemical instruments and methods, peptides containing affinity tags, etc., can solve the problems of unstable synthesis of short peptides, low fusion efficiency of carrier proteins, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Recombinant plasmids pGEX-6p-ERK-pTY, pGEX-6p-YAP1-p127, pcDNA-GFP-ERK-pTY, pcDNA-GFP-YAP1-p127, pcDNA-GFP-ERK-pep and pcDNA-GFP- Construction of YAP1-pep;
[0055] Design primers and introduce enzyme cutting sites EcoRI and XhoI:
[0056] ERK-pep-5:AATTCGACCATGATCACACAGGGTTCCTGACTGAATACGTGGCCACACGTTGGTAGC;
[0057] ERK-pep-3:TCGAGCTACCAACGTGTGGCCACGTATTCAGTCAGGAACCCTGTGTGATCATGGTCG;
[0058] YAP1-pep-5:AATTCACTCCACAGCATGTTCGAGCTCATTCGTCTCCAGCTTCTCTGCAGTTGTGAC;
[0059] YAP1-pep-3:TCGAGTCACAACTGCAGAGAAGCTGGAGACGAATGAGCTCGAACATGCTGTGGAGTG;
[0060] YAP1-p127-5:AATTCACTCCACAGCATGTTCGAGCTCATGACTCTCCAGCTTCTCTGCAGTTGTGAC;
[0061] YAP1-p127-3:TCGAGTCACAACTGCAGAGAAGCTGGAGAGTCATGAGCTCGAACATGCTGTGGAGTG;
[0062] ERK-pTY-5:AATTCGACCATGATCACACAGGGTTCCTGGACGAAGACGTGGCCACACGTTGGTAGC;
[0063] ERK-pTY-3: TCGAGCTACCAACGTGTGGCCACGTCTTCGTCCAGGAACCCTGTGTGATCATGGTCG;
[0064] The primers were annealed to form dimers, and the conditions for annealing the primers to form ...
Embodiment 2
[0066] Example 2: Expression and purification of fusion proteins GST-YAP1-p127 and GST-ERK-pTY
[0067] Ultrasonic disruption of bacteria: add 40ml PBS and 400μl PMSF to the bacteria, ultrasonic cracking; ultrasonic conditions: horn φ6, power 80%, total ultrasonic time 4min, ultrasonic 3s, interval 3s. Rotate and mix the lysed cells at 4°C for 30 minutes, then centrifuge at 10,000 rpm for 20 minutes, take the supernatant and add it to a new centrifuge tube to repeat centrifugation once, and collect the supernatant. Add 300 μl GST Sepharose to the lysed supernatant. Rotate and shake in a chromatographic cabinet at 4°C for 1 to 2 hours. Centrifuge at 4°C and 2000rpm for 2min, and discard the supernatant. Add PBS to the centrifuge tube with gel to 40ml, shake in a chromatographic cabinet at 4°C for 30min, centrifuge at 2000rpm at 4°C for 2min, discard the supernatant, add PBS and repeat twice. Transfer the gel to a 1.5ml EP tube, centrifuge at 2000rpm for 2min, discard the sup...
Embodiment 3
[0068] Example 3: Animal Immunization
[0069] 3.1 Mix the dialyzed antigen with Freund's complete adjuvant or Freund's incomplete adjuvant at a volume ratio of 1:1, and rotate and mix at 4°C for 2 hours until water-in-oil droplets are formed.
[0070] 3.2 Select healthy New Zealand white rabbits about 7 weeks old, and feed them in the animal room for 4 to 6 days to adapt to the environment.
[0071] In the first week, inject 1 mg of antigen GST-YAP1-p127 and Freund's complete adjuvant mixture at 4 points on the left and right thighs of rabbits;
[0072] In the fourth week, inject 1mg of antigen GST-YAP1-p127 mixed with Freund's incomplete adjuvant;
[0073] In the sixth week, inject 1mg of antigen GST-YAP1-p127 mixed with Freund's incomplete adjuvant;
[0074] In the eighth week, inject 1mg of antigen GST-YAP1-p127 mixed with Freund's incomplete adjuvant;
[0075] At the ninth week, blood was collected from the carotid artery. The collected blood was incubated at 37°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com