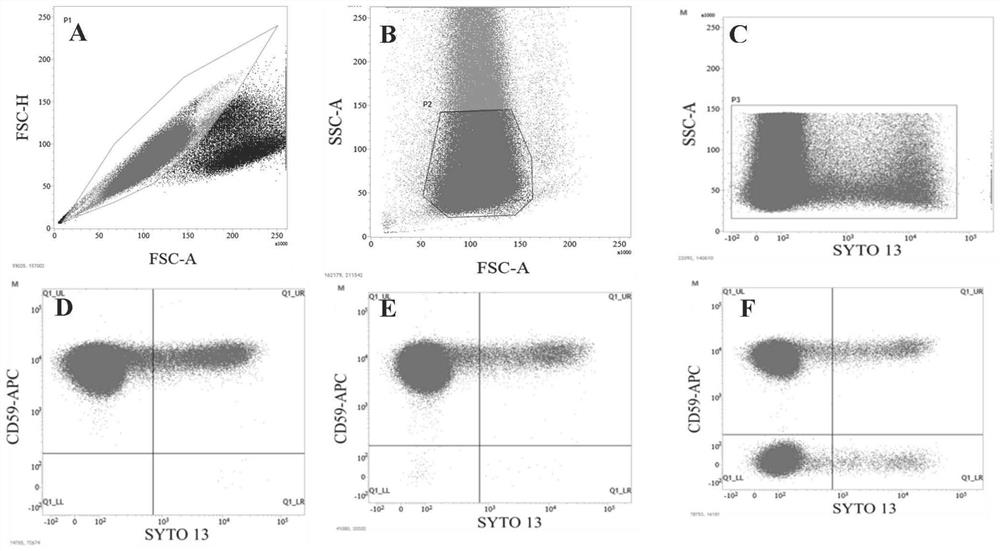
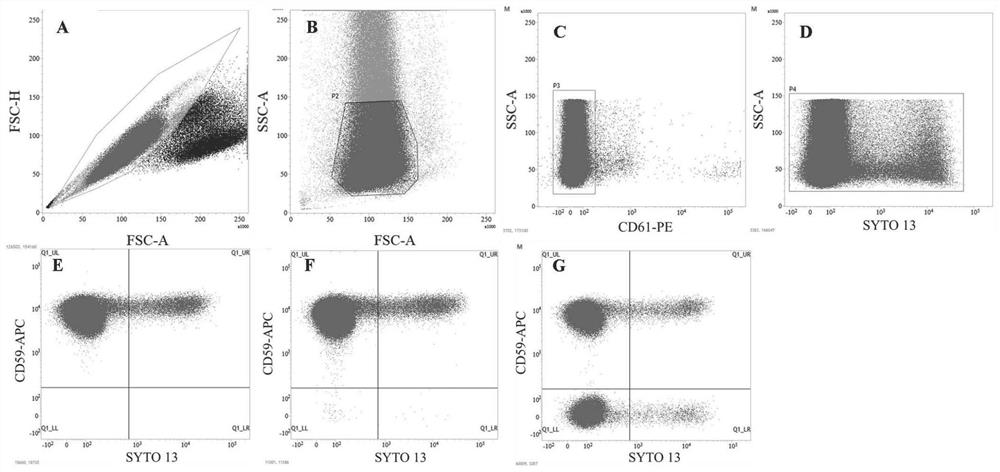
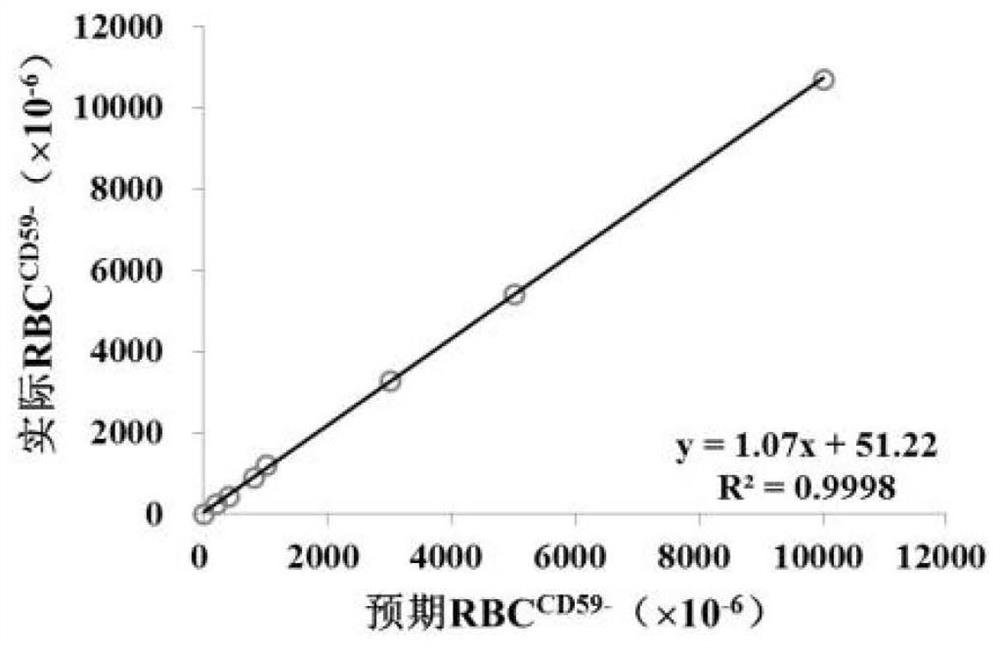
Flow cytometry detection method for Pig-a gene mutation test in rat body
A technology of flow cytometry and detection method, applied in the field of flow cytometry detection of rat peripheral blood Pig-a gene mutation test, can solve the problems of high cost, increased operation steps, high detection cost, etc., and achieve high sensitivity and stability High reliability, high stability, and cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] sample collection
[0036] Take 5-week-old male SD rats of SPF grade, weighing 150-160 g. The doses of test substance A were 0, 20, 40, 80, 160mg / kg.bw / d, and the solvent was pure water. The drug was administered orally for 28 consecutive days, and the samples were collected on the 1st, 14th, and 28th day before the test, respectively. Peripheral blood testing.
[0037] When collecting blood, wipe the rat tail with an alcohol cotton ball to fill the blood vessels in the tail, and use a syringe needle to puncture the blood vessels along the parallel direction of the blood vessels. Collect 50 μL of naturally flowing blood into a centrifuge tube (containing 10 μL heparin sodium solution), and mix immediately to prevent coagulation. After the blood collection, the animals should be disinfected and the bleeding should be stopped. The collected peripheral anticoagulated blood was stored in a refrigerator at 4°C and protected from light until testing.
[0038] Reagent prep...
Embodiment 2
[0054] sample collection
[0055] Take 5-week-old male SD rats of SPF grade, weighing 150-160 g. The exposure doses of test substance B were 20, 40 and 80 mg / kg.bw / d, and the solvent was pure water. The drug was administered orally for 3 consecutive days, and peripheral blood was collected on the 28th day of the experiment for testing.
[0056] When collecting blood, wipe the rat tail with an alcohol cotton ball to fill the blood vessels in the tail, and use a syringe needle to puncture the blood vessels along the parallel direction of the blood vessels. Collect 50 μL of naturally flowing blood into a centrifuge tube (containing 10 μL heparin sodium solution), and mix immediately to prevent coagulation. After the blood collection, the animals should be disinfected and the bleeding should be stopped. The collected peripheral anticoagulated blood was stored in a refrigerator at 4°C and protected from light until testing.
[0057] Reagent preparation
[0058] APC mouse anti-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com