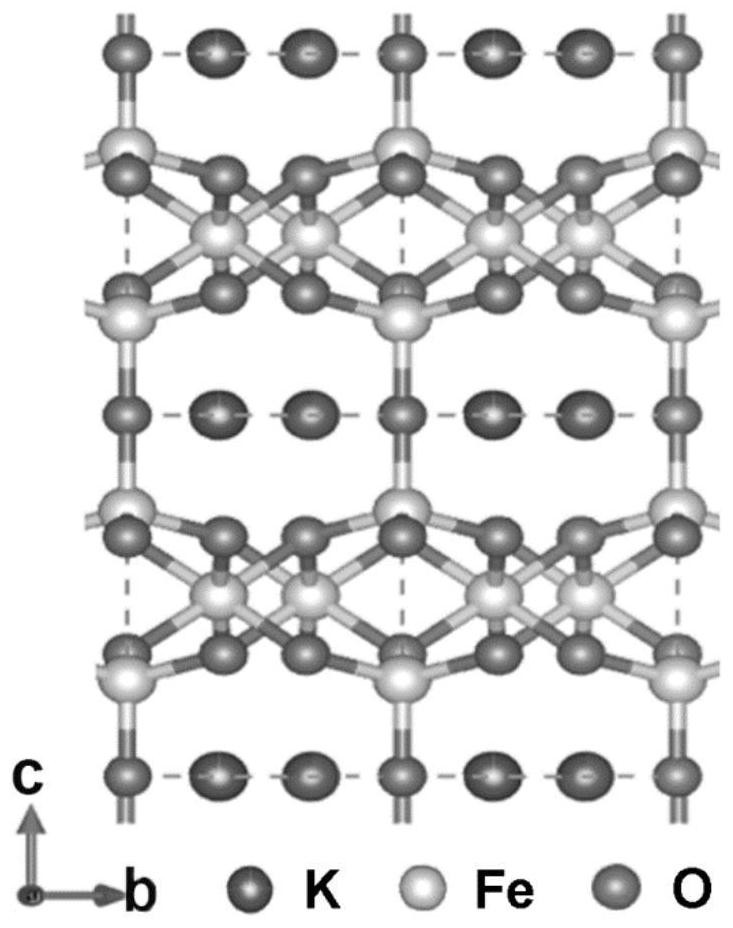
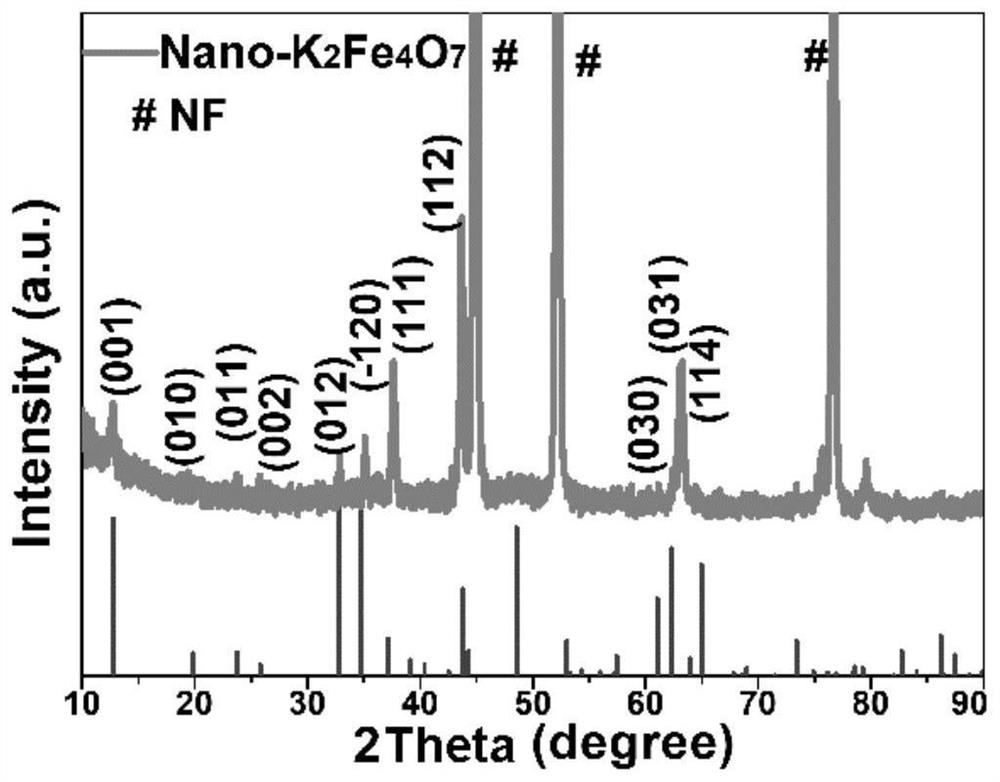
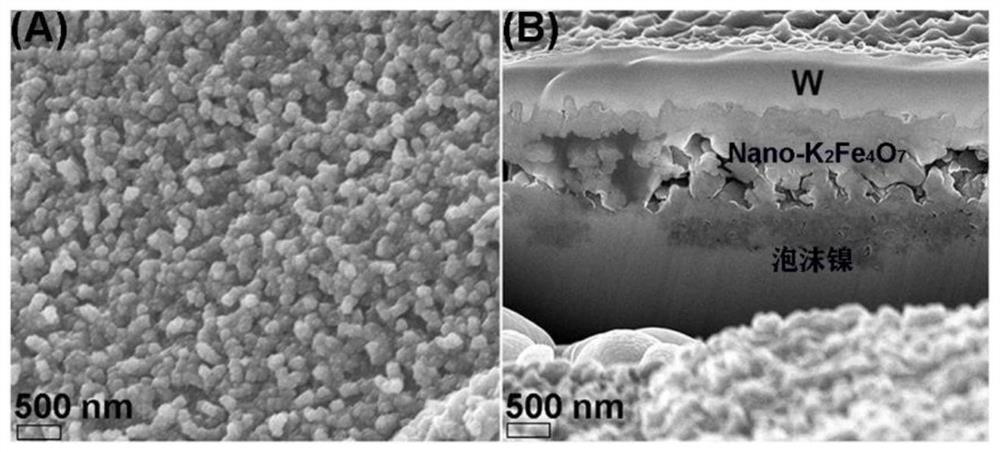
Foamed nickel-based Nano-K2Fe4O7 catalyst, preparation method and application of foamed nickel-based Nano-K2Fe4O7 catalyst in efficient electrocatalytic hydrolysis
A nano-k2fe4o7, catalyst technology, applied in the field of electrocatalytic material preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] First, cut out 1*9cm 2 Nickel foam sheet (thickness 1mm, width 1cm, length 9cm), ultrasonic 10 minutes in the dilute hydrochloric acid solution of 2.0M; Use deionized water and ethanol to carry out multiple washings to the nickel foam after acid treatment afterwards, then treat The final foamed nickel is placed in a dry box for subsequent use; then, the dried foamed nickel is put into a polyethylene reaction liner, and then dissolved with KOH (72g), Fe(NO 3 ) 3 9H 2 The aqueous solution (~32mL) of O (4.04g) was transferred to the PTFE liner with nickel foam; finally, the reaction kettle was placed in an oven at 240°C for 48 hours, and the foamed nickel-based material obtained was In situ loading with Nano-K 2 Fe 4 o 7 The foamed nickel-based catalytic material, marked as Nano-K 2 Fe 4 o 7 .
[0038] The electrocatalytic hydrolysis performance test was carried out on the catalytic material prepared by the above method, and the reaction system was a standard thre...
Embodiment 2
[0046] Same as embodiment 1, under the premise that the foamed nickel width and length are constant (the width of the foamed nickel is 1cm, and the length is 9cm), only the thickness of the foamed nickel introduced is adjusted, that is, the foamed nickel thickness is adjusted to 0.5mm, the resulting product is marked as Nano-K 2 Fe 4 o 7 -0.5mm. Under the condition of 1.0M KOH, the electrocatalytic properties of the obtained samples are as follows: Figure 8 Shown:
[0047] In the process of electrocatalytic oxygen evolution, when the overpotential is 520mV, the current density of the material reaches 1000mA / cm 2 ,Such as Figure 8 as shown in (A);
[0048] In the process of electrocatalytic hydrogen evolution, when the overpotential is 490mV, the current density of the material reaches -1000mA / cm 2 ,Such as Figure 8 (B) shown.
Embodiment 3
[0050] Same as Example 1, under the premise that the foamed nickel thickness and length are constant (the thickness of the foamed nickel is 1mm, and the length is 9cm), only the width of the foamed nickel introduced into the reaction system is adjusted, that is, the width is adjusted to 1.2 cm, the resulting product is labeled Nano-K 2 Fe 4 o 7 -1.2cm. Under the condition of 1.0M KOH, the electrocatalytic properties of the obtained samples are as follows: Figure 9 Shown:
[0051] In the process of electrocatalytic oxygen evolution, when the overpotential is 428mV, the current density of the material reaches 1000mA / cm 2 ,Such as Figure 9 as shown in (A);
[0052] In the process of electrocatalytic hydrogen evolution, when the overpotential is 422mV, the current density of the material reaches -1000mA / cm 2 ,Such as Figure 9 (B) shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com