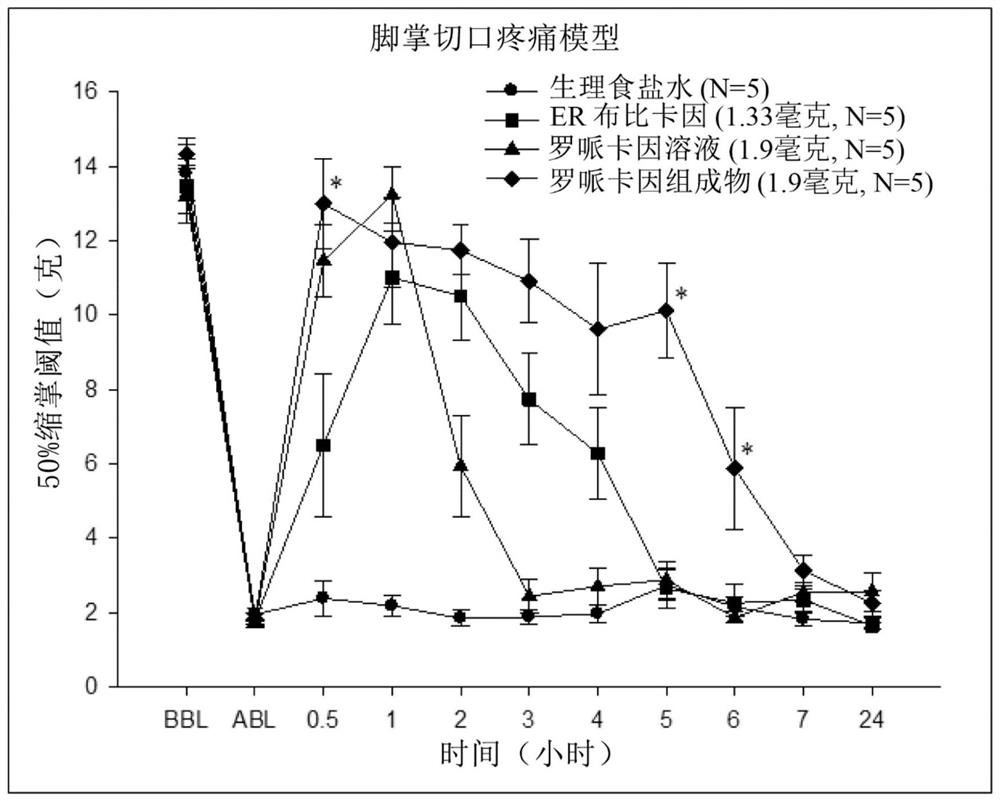
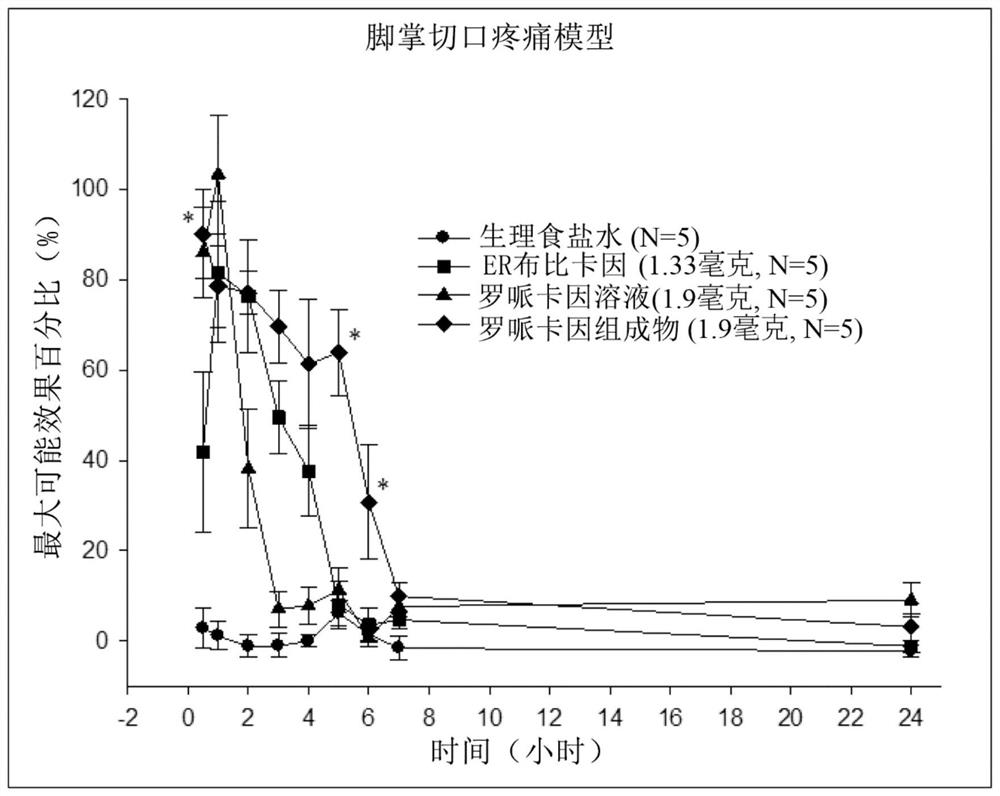
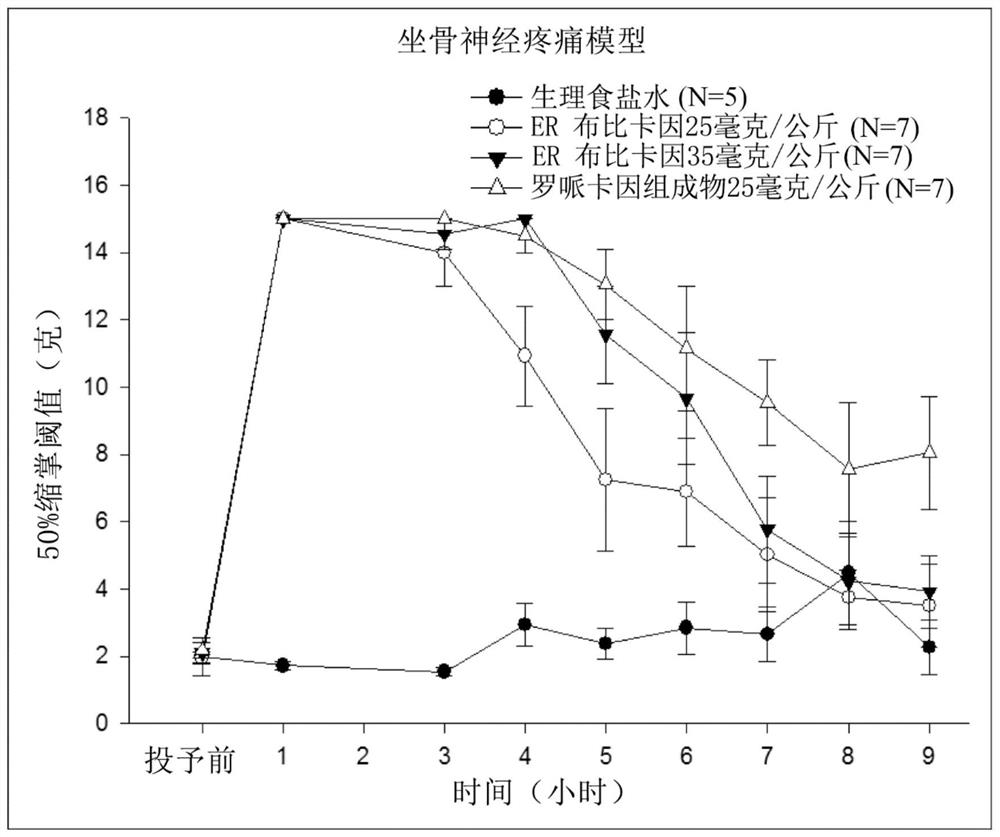
Sustained-release anesthetic compositions and methods of preparation thereof
A composition and anesthetic technology, applied in the field of sustained-release pharmaceutical composition, can solve problems such as lengthy steps and high production costs, and achieve the effect of avoiding inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Method for preparing ropivacaine composition from multiple phospholipids
[0062] Phospholipids, including DLPC, DMPC, DPPC, DOPG, DOPC, DOPS, DOPA, Egg PC, Egg PE, POPE, cardiolipin, and DMPA were purchased from NOF Corporation (Tokyo, Japan), or Lipoid GmbH (Ludwigshafen, Germany) ); Cholesterol was purchased from Sigma-Aldrich (Darmstadt, Germany) or Dishman Pharmaceuticalsand Chemicals (Gujarat, India), and ropivacaine was purchased from Apollo Scientific (Cheshire, UK) or Dishman Pharmaceuticals and Chemicals; all others The chemicals were purchased from Sigma-Aldrich.
[0063] In order to prepare lipid cakes, ropivacaine was mixed with different lipid mixtures in Table 1 at a drug relative phospholipid ratio (D:PL) of 1.458 micromole / micromole, ie, phospholipid:cholesterol:ropivaca Because=2:1:2.9. Mix lipid and ropivacaine, and dissolve in tert-butanol or tert-butanol-water co-solvent (volume ratio 1:1) to form a liquid phase structure. After freezing each liquid-p...
Embodiment 2
[0067] Characterization of ropivacaine composition
[0068] The sample prepared in Example 1 will be tested for its association efficiency by the method described below. 200 microliters of ropivacaine composition was centrifuged at 3000xg for 5 minutes at 4°C. After removing the supernatant, obtain the separated lipid substrate mixture and resuspend it to 200 microliters; use a test drug solution of known concentration (such as ropivacaine) to establish a reference standard for its absorbance. An ultraviolet / visible light spectrophotometer was used to measure the amount of ropivacaine drug in the original ropivacaine composition and the separated lipid substrate mixture. The association efficiency represents the ratio of the separated lipid substrate mixture to the amount of drug in the ropivacaine composition. The D:PL lipid cake of the separated lipid matrix complex is calculated by multiplying the D:PL of the lipid cake by the association efficiency, which is called "final D...
Embodiment 3
[0077] Preparation of ropivacaine composition containing different phospholipids
[0078] The sources of phospholipids, cholesterol, ropivacaine, and all other chemicals are detailed in Example 1.
[0079] In order to prepare a lipid cake, ropivacaine and different lipid mixtures are mixed at a molar ratio of phospholipid:cholesterol:ropivacaine=2:1:2.9, where the phospholipid is DMPC and one of the other phospholipids in Table 2. The molar ratio of DMPC: a kind of other phospholipid in the phospholipid is 1.8:0.2. After the lipid is mixed with ropivacaine, it is dissolved in tert-butanol to form a liquid phase structure. After each liquid phase structure sample is frozen for 30 to 60 minutes, freeze-drying is continued until the next day to obtain a lipid cake.
[0080] The lipid cake was hydrated with 50 mM histidine buffer (pH 6.5) at room temperature to form a ropivacaine composition, and then the association efficiency and particle size distribution were measured.
[0081] In t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com