Full dry-mixing infant formula milk powder and preparation method of full dry-mixing infant formula milk powder
A technology for infant formula and milk powder, applied in the direction of milk preparations, dairy products, applications, etc., can solve the problems of unfavorable long-term storage, etc., and achieve the effect of being suitable for long-term storage, with small limitations, and reducing production costs and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Another object of the present invention is to provide a method for preparing dry-mixed infant formula milk powder, including the following steps:
[0041] 1) Material preparation: the main materials and auxiliary materials are unpackaged and sterilized by ultraviolet light, and the main materials and auxiliary materials are weighed according to the formula;
[0042] 2) Pre-mixing: add 1-10 parts by weight of skimmed milk powder or desalted whey powder to the auxiliary materials for pre-mixing, and mixing uniformly to obtain a pre-mix;
[0043] 3) Total mixing: the premix obtained in step 2) is uniformly mixed with other main materials to obtain the product;
[0044] 4) Vacuum: the product obtained in step 3) is weighed and divided into bags, and the package is vacuumed until the residual oxygen content is ≤ 2%.
[0045] The preparation method of the dry-mixed infant formula milk powder provided by the present invention has good uniformity of the milk powder, the active ingredient...
Embodiment 1
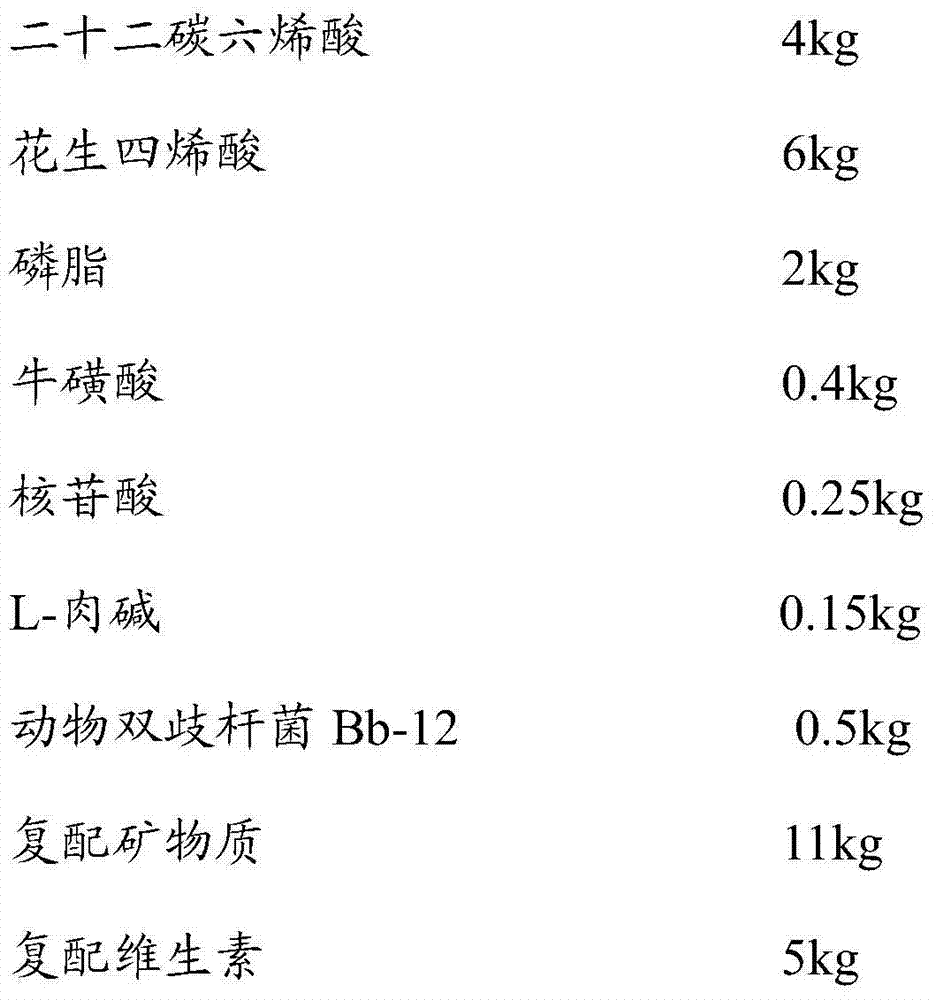
[0049] Example 1: Preparation of infant formula milk powder suitable for 0-6 months
[0050] An infant formula milk powder whose formula is as follows:
[0051]
[0052]
[0053] Among them, the compound minerals are composed of materials mixed in weight percentage: calcium carbonate 16.20%, calcium phosphate 8.4%, calcium hydroxide 8.1%, potassium dihydrogen phosphate 7.2%, potassium hydroxide 3.61%, potassium citrate 6.32%, chlorine Potassium 20.43%, Magnesium Chloride 3.72%, Sodium Chloride 8.93%, Sodium Citrate 7.6%, Ferrous Sulfate 2.5%, Zinc Sulfate 0.7%, Copper Sulfate 0.06%, Manganese Sulfate 0.001%, Potassium Iodide 0.007%, Sodium Selenite 0.001%, lactose 6.2%;
[0054] The compound vitamin is composed of the following materials in weight percentage: L-sodium ascorbate 21.97%, choline bitartrate 15.6%, inositol 7.97%, nicotinamide 1.33%, D-calcium pantothenate 0.84%, D-biotin 0.006% , Cyanocobalamin 0.0003%, thiamine hydrochloride 0.21%, pyridoxine hydrochloride 0.20%, rib...
Embodiment 2
[0065] Example 2: Preparation of infant formula milk powder suitable for 6-12 months old
[0066]
[0067]
[0068] Among them, the compound mineral is composed of the following materials in weight percentage: calcium carbonate 19.20%, calcium phosphate 7.4%, calcium hydroxide 7.3%, potassium dihydrogen phosphate 6.9%, potassium hydroxide 3.61%, potassium citrate 6.13% , Potassium chloride 19.15%, magnesium chloride 3.84%, sodium chloride 3.91%, sodium citrate 6.7%, ferrous sulfate 3.9%, zinc sulfate 0.7%, copper sulfate 0.07%, manganese sulfate 0.001%, potassium iodide 0.007%, selenium Sodium 0.001%, lactose 11.18%.
[0069] The compound vitamin is composed of the following materials in weight percentage: 20.25% sodium L-ascorbate, 14.9% choline bitartrate, 7.75% inositol, niacinamide 1.31%, D-calcium pantothenate 0.86%, D-biotin 0.006% , Cyanocobalamin 0.0003%, Thiamine Hydrochloride 0.21%, Pyridoxine Hydrochloride 0.20%, Riboflavin 0.23%, Folic Acid 0.04%, dl-α-Tocopherol Acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com