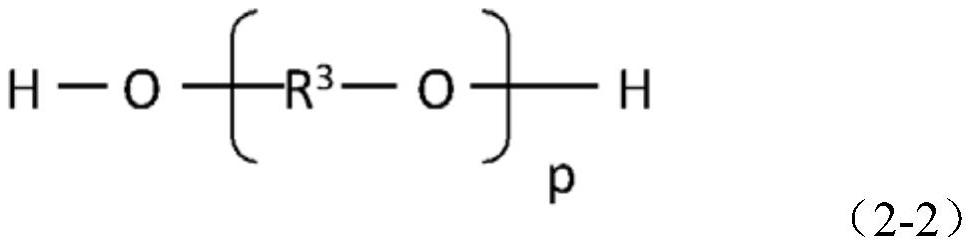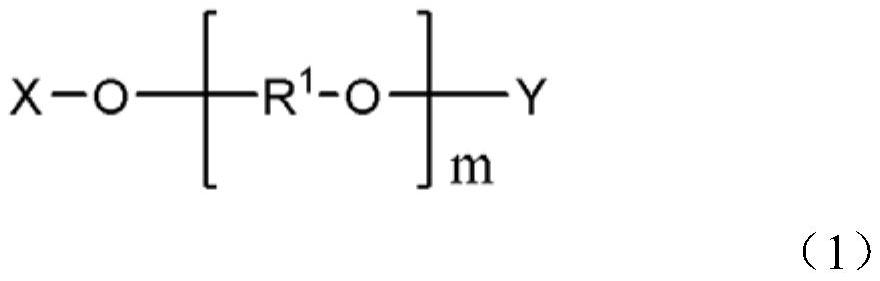Method for producing carbodiimide compound
A technology of carbodiimide and compounds, which is applied in the field of preparation of carbodiimide compounds, can solve problems such as complicated procedures and achieve high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] 1. The preparation method of carbodiimide compound
[0055] The preparation method of the carbodiimide compound of the present embodiment includes: adding an inorganic alkali metal compound (B) and a compound (D-1) selected from a phase transfer catalyst (C), the following general formula (2-1) and A carbodiimide production step of reacting an aliphatic tertiary isocyanate compound (A) in the presence of at least one compound (D-2) represented by the following general formula (2-2).
[0056] [chemical formula 4]
[0057]
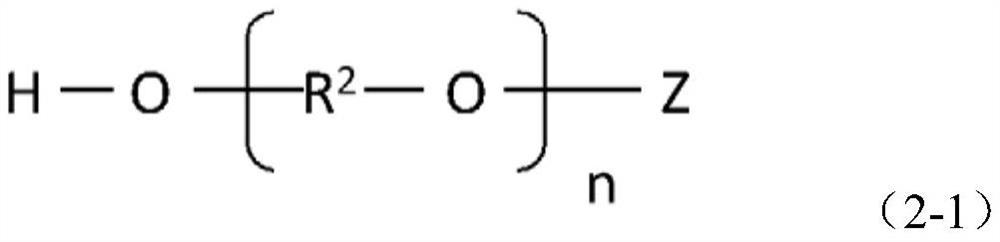
[0058] (In formula (2-1), Z is methyl, ethyl, propyl, butyl or phenyl. R 2 It is an alkylene group having 2-3 carbon atoms. n is an integer of 2-500. )
[0059] [chemical formula 5]
[0060]
[0061] (In formula (2-2), R 3 It is an alkylene group having 2-3 carbon atoms. p is an integer of 2-500. )
[0062] As mentioned above, an inorganic alkali metal compound normally functions as a catalyst which accelerates the trimerization reactio...
Embodiment 1
[0240] 100 g of tetramethylxylylene diisocyanate as an aliphatic tertiary isocyanate compound (A), 0.5 g of potassium hydroxide (KOH) as an inorganic alkali metal (B) and 1.0 g of a phase transfer catalyst (C) 18-Crown-6-ether was added to a 300ml reaction vessel equipped with a reflux tube and a stirrer, stirred at 175°C under a nitrogen stream, and reacted until the NCO% measurement was 3.74%. The synthesis time was 5 hours.
[0241] In addition, the NCO% value of 3.74% refers to the case where 11 tetramethylxylylene diisocyanate undergoes decarboxylation condensation to produce a carbodiimide compound with a degree of polymerization of 10 (NCO groups at both ends). The content (mass %) of the NCO group in the carbodiimide compound below. With this value of 3.74% as the target value, the above reaction was carried out until the measured value of NCO% reached the target value.
[0242] As a result of analyzing the obtained isocyanate-terminated polytetramethylxylylenecarbod...
Embodiment 2
[0247] 100 g of 3-isopropenyl-α,α-dimethylbenzyl isocyanate as the aliphatic tertiary isocyanate compound (A), 0.5 g of potassium hydroxide (KOH) as the inorganic alkali metal (B) and 1.0 g as the phase The 18-crown-6-ether of transfer catalyst (C) is added in the 300ml reaction vessel that is equipped with reflux tube and stirrer, under nitrogen stream, stirs at 175 ℃, reacts until by infrared absorption (IR) spectrometry and wavelength 2200 -2300cm -1 The absorption of the isocyanate groups disappears (until the NCO% is 0%). The synthesis time was 20 hours.
[0248] The resulting bis(3-isopropenyl-α,α-dimethylbenzyl)monocarbodiimide was analyzed, and it was confirmed by infrared absorption (IR) spectroscopy that the wavelength was 2118cm -1 Around the absorption peak of the carbodiimide group. Absorption wavelength due to isocyanurate could not be confirmed, wavelength 1710cm -1 Left and right, wavelength 1411cm -1 The left and right absorption peaks, the absorption wav...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com