Application of 6-dimethylaminoquinoline aromatic ethylene derivative to preparation of drugs for resisting drug-resistant bacteria
A technology of dimethylaminoquinoline aromatic vinyl and drug-resistant bacteria is applied in the directions of antibacterial drugs, resistance to vector-borne diseases, pharmaceutical formulations, etc., and can solve the problem of not finding 6-dimethylaminoquinoline aromatic vinyl derivatives and the like , to achieve the effect of reducing bacterial resistance, inhibiting growth and reproduction, and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Compounds 1-12
[0049] The preparation methods of compounds 1-12 (Table 1) have been disclosed in the patent (WO2006078754).
Embodiment 2
[0050] Example 2 Antibacterial activity screening
[0051] In this example, the micro broth dilution method was used to determine the minimum inhibitory concentration MIC (μg / mL) value of 6-dimethylaminoquinoline aromatic vinyl derivatives, according to the broth described in the Clinical and Laboratory Standards Institute (CLSI) guidelines The microdilution procedure determines the minimum inhibitory concentration (MIC) of the test compound. Among them, the test compounds are shown in Table 1.
[0052] Table 1 Test compounds
[0053]
[0054] The implementation steps are as follows:
[0055] (1) Preparation of culture medium and antibacterial drug stock solution: autoclave the prepared MH broth medium at 121°C for 30 minutes and then cool; dissolve the test compound in DMSO to prepare a stock solution of 3.2 mg / mL, and use 0.22 The μm membrane filter sterilizes for later use.
[0056] (2) Activation and expansion of test bacteria: spread the frozen test bacteria on a TSB agar plate,...
Embodiment 3
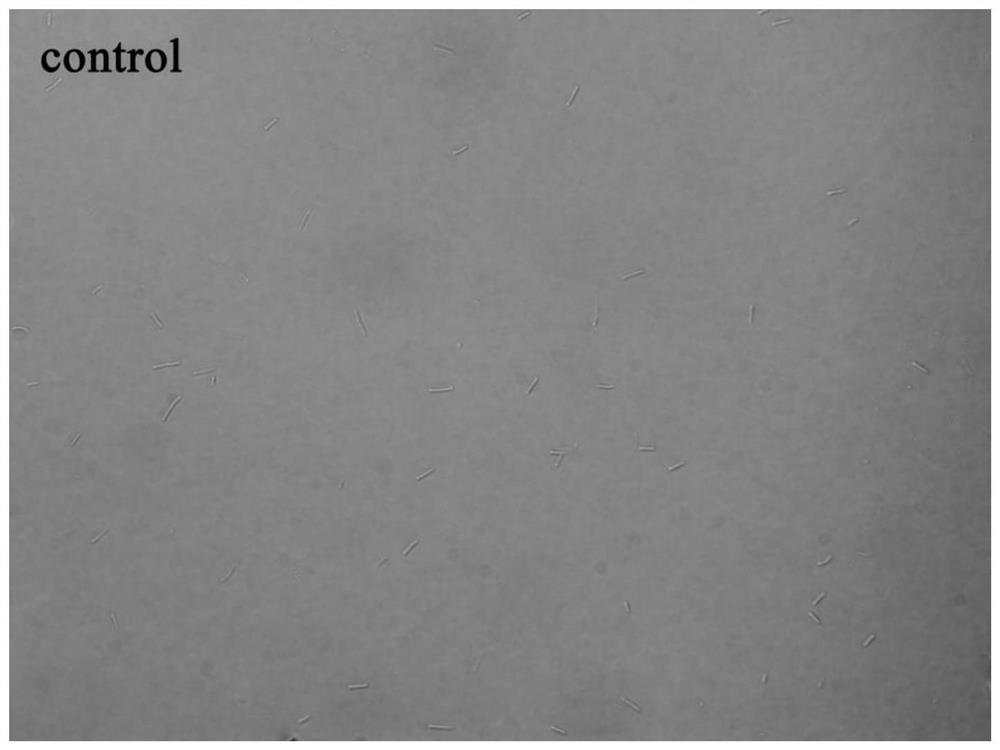
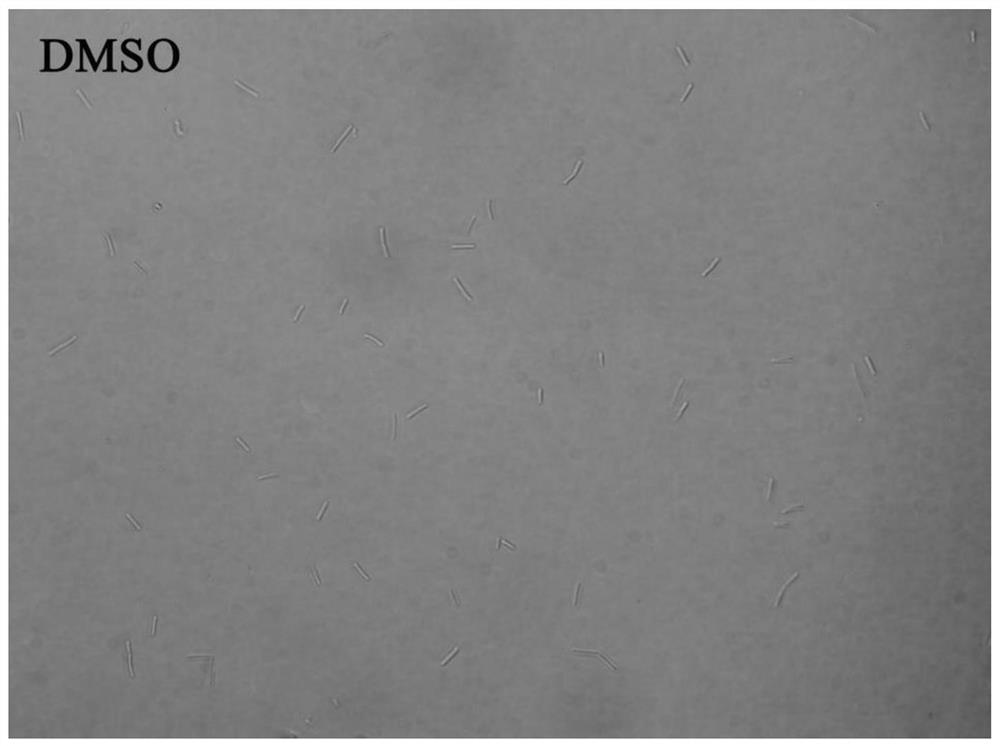
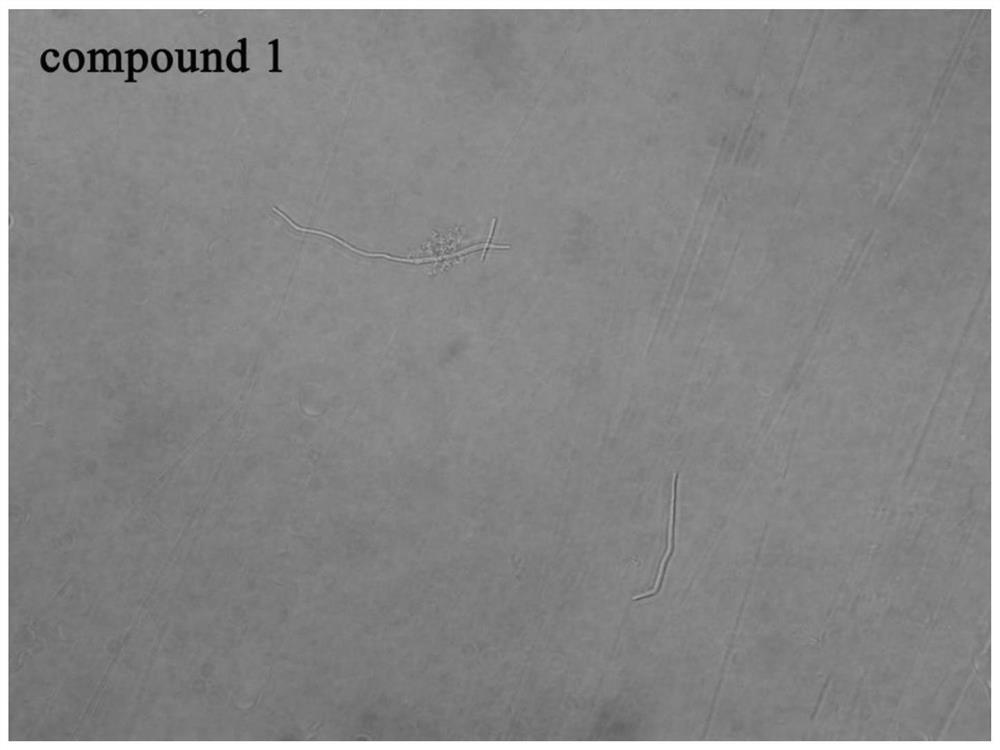
[0066] Example 3 Morphological study of bacteria
[0067] In this example, an Olympus IX71 inverted fluorescence microscope was used to observe the growth morphology of Bacillus subtilis under the action of the 6-dimethylaminoquinoline aromatic vinyl derivative.
[0068] The test compounds are compounds 1-12 in Table 1; the test bacteria choose B.subtilis 168; because the compound concentration is the MIC value, the growth of the bacteria is effectively inhibited, so the experimental concentration adopts 0.5×MIC to obtain a certain concentration Observe the bacterial suspension.
[0069] The implementation steps are as follows:
[0070] (1) Dilute the expanded bacterial solution with sterilized MH broth medium to a concentration of about 5×10 5 CFU / mL.
[0071] (2) Add the diluted bacterial solution and compound stock solution to the sterilized 5mL centrifuge tube, the total volume is set to 1mL, and the final concentration of the compound is the 0.5×MIC value of each compound inhibiti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com