African swine fever virus wild strain and vaccine strain identification and detection kit
An African swine fever virus and wild virus strain technology is applied in the field of identification and detection kits for African swine fever virus wild virus strains and vaccine strains. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Preparation and Application of Triple Fluorescent PCR Identification Detection Kit for African Swine Fever Virus Wild Strain and Vaccine Strain
[0087] 1. Preparation of triple fluorescent PCR identification kit for African swine fever virus wild strain and vaccine strain
[0088] 1. Synthesis of Primers and Probes
[0089] The following primers and probes were artificially synthesized:
[0090] Primer P72F3: 5'-GTTTCGGTACGCATTCTTTGTG-3';
[0091] Primer P72R3: 5'-CGGATATGACTGGGACAACCA-3';
[0092] Probe P72P3: 5'-Cy5-ATCTACAAGCGTGTAAACGGCGCCC-BHQ-3-3'
[0093] The 5'-end of the probe P72P3 is labeled with the fluorescent reporter group Cy5, and the 3'-end is labeled with the fluorescent quencher group BHQ-3.
[0094] CD2vF2: 5'-CCCAATATCCCGCCATTATCT-3'
[0095] CD2vR2: 5'-AAATGAAGACCAGCTTGAAAGGTT-3'
[0096] CD2vP2: 5'-VIC-TTCGCTTATTCACGTAGATAG-BHQ1-3'
[0097] The 5'-end of the probe CD2vP2 is labeled with the fluorescent reporter group VIC, and the...
Embodiment 2
[0118] Example 2. Detection of Sensitivity of African Swine Fever Virus Field Strain and Vaccine Strain Triple Fluorescence PCR Differentiation Detection Kit
[0119] The sensitivity test was performed using the kit prepared in Example 1.
[0120] 1. Preparation of template DNA
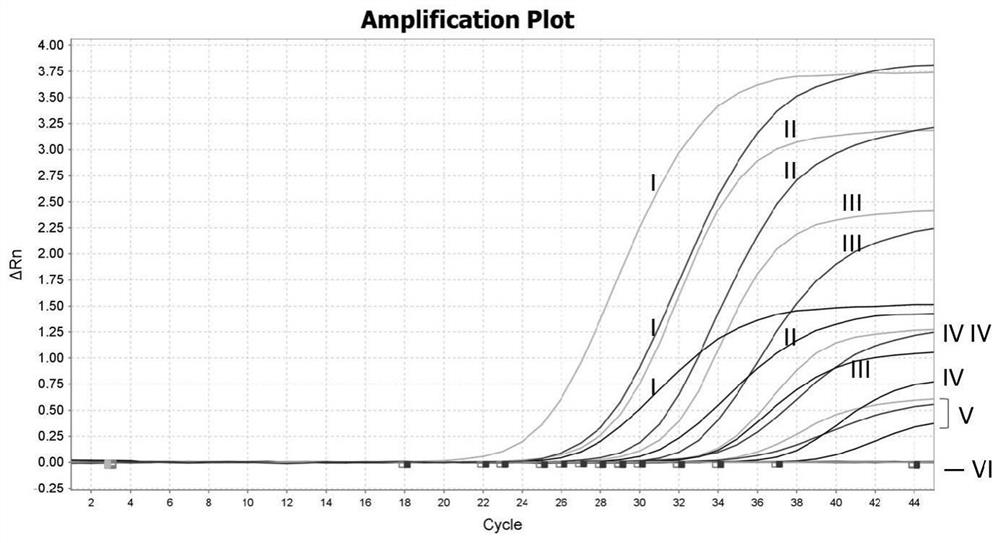
[0121] With the step 1 of embodiment 1, prepare final concentration respectively and be 3 * 10 6 Copy / μL B646L gene, EP402R gene and mCherry gene positive standard, take 100 μL each into a 1.5mL centrifuge tube, and obtain a final concentration of 1×10 6 Copies / μL of B646L gene, EP402R gene and mCherry gene positive standard mixture. Carry out 10-fold serial dilutions to the positive standard mixture with double distilled water, prepare dilution I respectively (concentration is 1×10 6 copy / μL), diluent II (each concentration is 1×10 5 copy / μL), diluent III (concentration is 1×10 4 copy / μL), diluent IV (concentration is 1×10 3 copy / μL), diluent V (each concentration is 1×10 2 copies / μL) and dilu...
Embodiment 3
[0135] Example 3. Detection of specificity of African swine fever virus wild strain and vaccine strain identification kit
[0136] The kit prepared in Example 1 was used for specific detection.
[0137] 1. Sample processing
[0138] Take 200 μL each of the virus solutions of African swine fever virus LNSY wild strain, ΔCD2vΔMGF vaccine candidate strain, CSF virus vaccine strain, and foot-and-mouth disease virus vaccine strain, and use a commercial virus nucleic acid extraction kit (Qiagen) to extract nucleic acid.
[0139] 2. Fluorescent PCR detection
[0140] Prepare the reaction master mix. The 25 μL reaction system contains 12.5 μL of 2×PCR reaction buffer and 10.5 μL of double distilled water.
[0141] WT tube: In a 0.2mL centrifuge tube, add 2μL of African swine fever virus LNSY wild strain nucleic acid, and then add 23μL of premix.
[0142] Vac tube: In a 0.2mL centrifuge tube, add 2μL of African swine fever virus ΔCD2vΔMGF vaccine candidate strain nucleic acid, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com