Preparation method and product thereof for fabric waterproof and darkening finishing agent
A technology of finishing agent and fabric, which is applied in the field of preparation of fabric waterproof and deepening finishing agent, which can solve the problems of surface color floating and poor washing fastness, poor waterproof effect of fabrics, and large amount of dyeing and chemical materials, etc., and achieves significant effective fluorine Alkyl shielding effect, excellent waterproof and deepening effect, and easy emulsification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
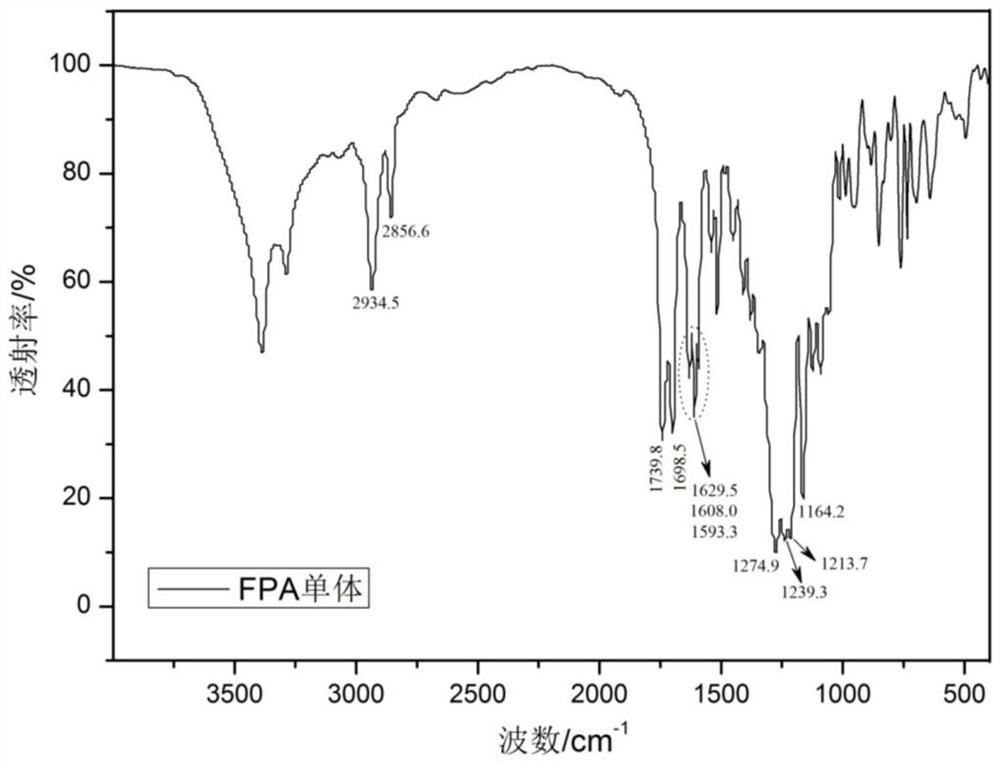
[0038] (1) Preparation of p-acryloyloxybenzoic acid: in a three-necked flask equipped with magnetic stirring, reflux condenser and thermometer, add 6.9 grams of p-carboxybenzoic acid, 4.0 grams of sodium hydroxide, and 50 grams of deionized water in sequence; Subsequently, 4.5 grams of acryloyl chloride was dissolved in 8 grams of acetone solvent, and at room temperature, was added dropwise to the above reaction solution through a constant pressure dropping funnel, and continued to react for 5 hours at room temperature after the drop was completed; Add a hydrochloric acid solution with pH=3 to the above reaction system, adjust the pH value of the solution to neutral, filter with suction, wash the obtained solid with deionized water at 50°C for 3 times, and then put the solid in an oven to dry for 2 hours to obtain a white solid in the form of intermediate The product p-acryloyloxybenzoic acid;
[0039] (2) Preparation of tridecafluorooctyl p-hydroxybenzoate: In a three-necked ...
Embodiment 2
[0054] (1) Preparation of p-acryloyloxybenzoic acid: same as Example 1.
[0055] (2) Preparation of trifluorooctyl p-hydroxybenzoate: same as Example 1.
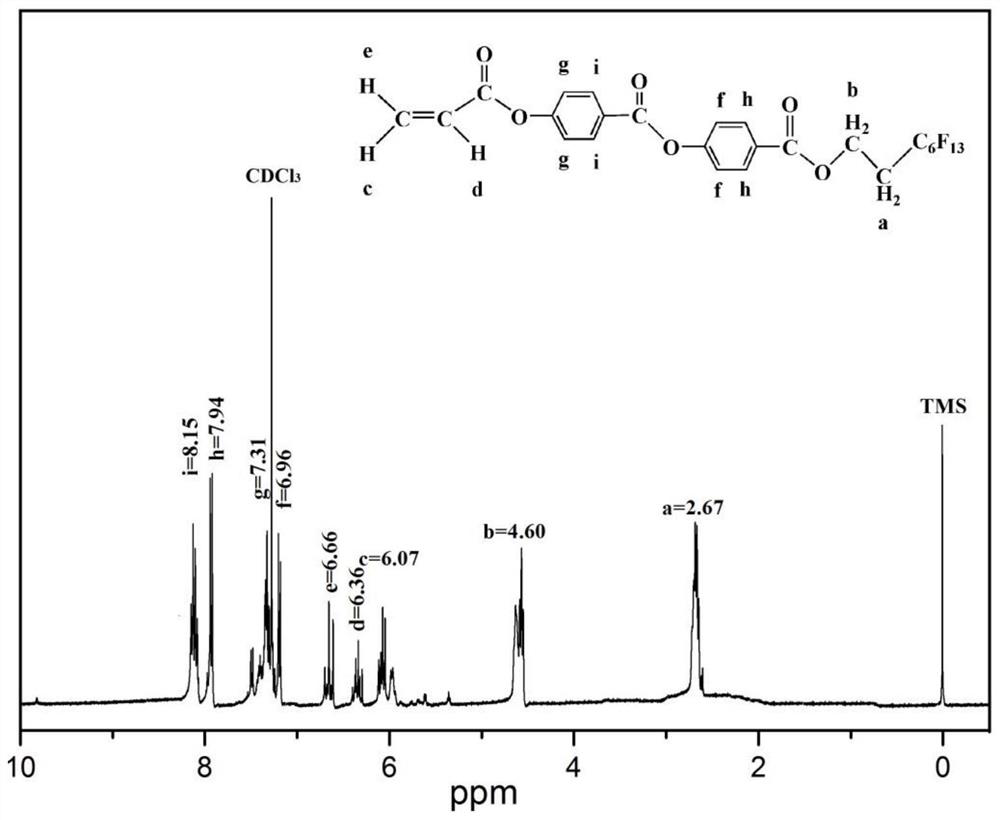
[0056] (3) Preparation of 2-(perfluorohexyl)ethyl-4-[4-(acryloyloxy)-benzoyloxy]benzoate (FPA): Same as Example 1.
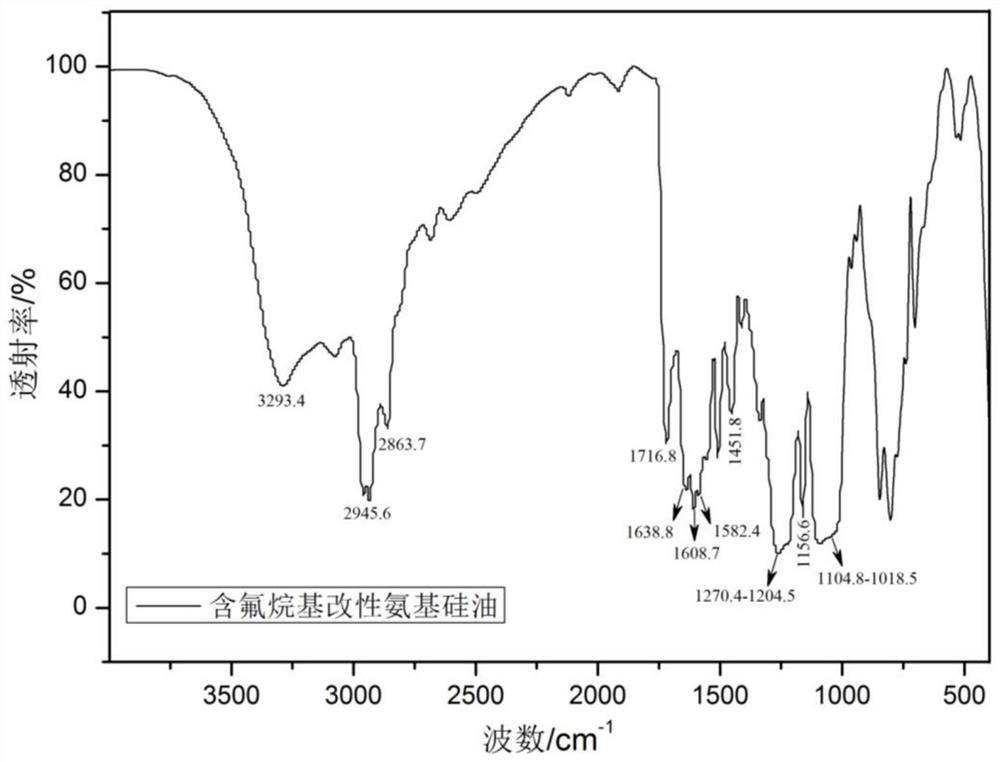
[0057] (4) Preparation of fluorine-containing alkyl-modified amino silicone oil: In a reactor equipped with a thermometer, reflux condenser and magnetic stirring, add 15 ml of tetrahydrofuran and 8.95 grams of amino silicone oil, and heat up the reaction solution to 60°C. 2-(perfluorohexyl)ethyl-4-[4-(acryloyloxy)-benzoyloxy]benzoate functional monomer was dissolved in 5 milliliters of tetrahydrofuran solvent, and the Add it dropwise to the above reaction system, and continue to react for 5 hours after the drop; subsequently, dissolve 1.9 grams of vinyltriethoxysilane in 5 milliliters of tetrahydrofuran solvent, and add it dropwise to the above reaction system through a constant pressure dropping funnel, Con...
Embodiment 3
[0061] (1) Preparation of p-acryloyloxybenzoic acid: same as Example 1.
[0062] (2) Preparation of trifluorooctyl p-hydroxybenzoate: same as Example 1.
[0063] (3) Preparation of 2-(perfluorohexyl)ethyl-4-[4-(acryloyloxy)-benzoyloxy]benzoate (FPA): Same as Example 1.
[0064] (4) Preparation of fluorine-containing alkyl-modified amino silicone oil: In a reactor equipped with a thermometer, reflux condenser and magnetic stirring, add 15 ml of tetrahydrofuran and 8.95 grams of amino silicone oil, and the temperature of the reaction solution is raised to 60 ° C. 2-(perfluorohexyl)ethyl-4-[4-(acryloyloxy)-benzoyloxy]benzoate functional monomer was dissolved in 15 milliliters of tetrahydrofuran solvent, and the Add it dropwise to the above reaction system, and continue to react for 5 hours after the drop; subsequently, dissolve 1.9 g of vinyltriethoxysilane in 5 ml of tetrahydrofuran solvent, and add it dropwise to the above reaction system through a constant pressure dropping f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com