Collagen targeting polypeptide probe containing aromatic amino acid as well as preparation method and application of collagen targeting polypeptide probe
A probe and peptide sequence technology, applied in the field of collagen detection, can solve the problems of tissue damage, difficulty in accurate quantification, etc., and achieve the effect of reducing difficulty and cost, broad application prospects, and good fluorescence and luminescence performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 polypeptide probe
[0043] 1. Design of peptide probes
[0044] The polypeptide probe sequences designed in this embodiment are: FAM-(Gly-Pro-Hyp) 3 -Gly-Tyr-Hyp-(Gly-Pro-Hyp) 4 、FAM-(Gly-Pro-Hyp) 3 -Gly-Phe-Hyp-(Gly-Pro-Hyp) 4 、FAM-(Gly-Pro-Hyp) 3 -Gly-Asp-Hyp-(Gly-Pro-Hyp) 4 、FAM-(Gly-Pro-Hyp) 3 -Gly-Ala-Hyp-(Gly-Pro-Hyp) 4 、FAM-(Gly-Pro-Hyp) 2 -Gly-Tyr-Hyp-Gly-Pro-Hyp-Gly-Tyr-Hyp-(Gly-Pro-Hyp) 3 、FAM-(Gly-Pro-Hyp) 2 -Gly-Ala-Hyp-Gly-Pro-Hyp-Gly-Ala-Hyp-(Gly-Pro-Hyp) 3 ;
[0045] Control peptide probe sequence: FAM-Pro 3 -Gly 3 -Hyp 3 -Pro-Gly-Hyp 2 -Pro 2 -Gly 3 -Hyp 3 -Pro 2 -Gly and FAM-(Gly-Pro-Hyp) 3 -Gly-Pro-Hyp-(Gly-Pro-Hyp) 4 , wherein FAM is carboxyfluorescein.
[0046] 2. Solid phase synthesis of peptide sequences
[0047] (1) Add 100 mg of Rink ammonia resin into a reactor with a sieve plate, and use 5 mL of dichloromethane to swell the resin;
[0048] (2) Remove the N-terminal Fmoc protecting group fr...
Embodiment 2
[0065] Example 2 Polypeptide Probe Targeted Binding Ability to Denatured Collagen
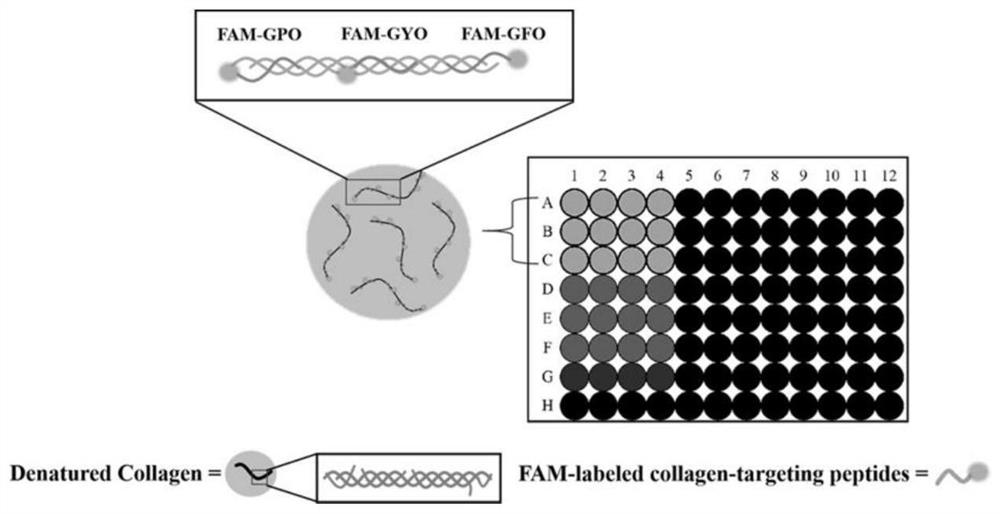
[0066] A 1 mg / mL gelatin solution was prepared in 10 mM PBS (pH 7.4) buffer at 70°C, added to a 96-well plate and air-dried. After film formation, block with 100 μL of 1% BSA blocking solution prepared in 10 mM PBS (pH 7.4) for 1 h, discard the solution in the well, wash with PBS 3 times, 3 min each time;
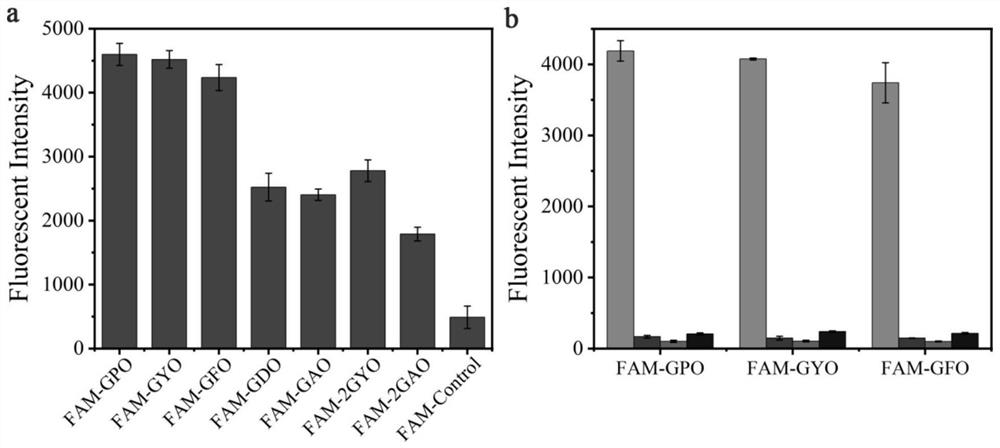
[0067] Take the polypeptide probes FAM-GPO, FAM-GYO, FAM-GFO, FAM-GDO, FAM-GAO, FAM-2GYO, FAM-2GAO and FAM-Control prepared in Example 1 respectively in 10mM PBS (pH7.4) Prepare a 20 μM peptide probe solution in 20 μM; add 70 μL of the peptide probe solution to a 96-well plate, and incubate at 4° C. for 4 h to allow it to bind to the gelatin membrane. Before use, the peptide probe solution was heated at 80 °C for 15 min to fully melt, and then immediately quenched in ice water for 30 s. The well plate was washed 3 times with 400 μL, 10 mM PBS (pH 7.4), 5 min each time. The fluorescence in...
Embodiment 3
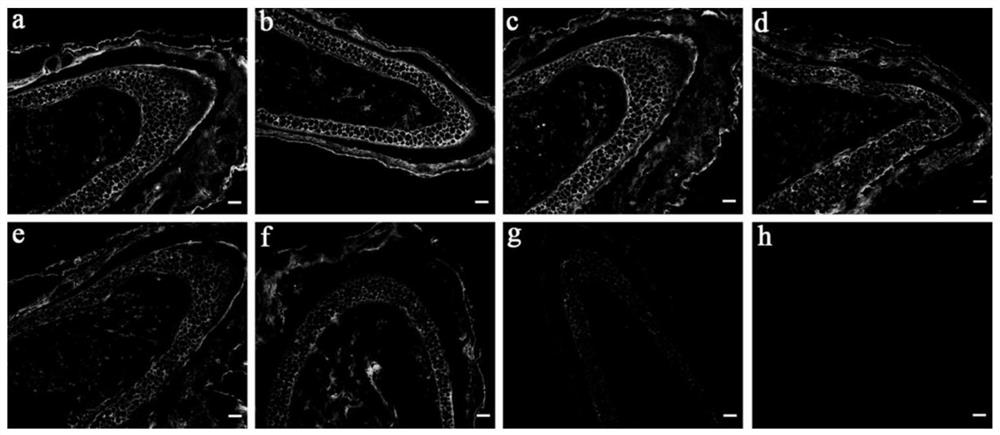
[0069] Example 3 Peptide Probes FAM-GYO and FAM-GFO Targeted Binding Specificity to Denatured Collagen
[0070] The evaluation of the binding ability of the polypeptide probe disclosed in the present invention to denatured collagen, pepsin, trypsin and hemoglobin was carried out according to the experimental scheme described in Example 2: the collagen dissolved in 0.5M acetic acid was heated at 70°C for 15min The solution (type I) was denatured, and 1 mg / mL denatured collagen (type I), hemoglobin, pepsin and trypsin solutions were added to a 96-well plate and air-dried. The orifice plate was washed 3 times with 10 mM PBS (pH 7.4), 3 min each time, and the plate was shaken and patted dry. The FAM-GPO, FAM-GYO and FAM-GFO solutions were heated at 80 °C for 15 min before use and immediately quenched in ice water for 30 s. Add 70 μL (20 μM) of the polypeptide probe solution (prepared as in Example 1) on the protein membrane, and incubate at 4° C. for 4 h. After the binding, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com