Preparation method of metrafenone
A technology of metrafenone and chlorobenzene, applied in the field of organic synthetic chemistry, can solve problems such as low total yield, difficult purification and separation, and difficult industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047] Embodiment: the concrete steps of the preparation method of a kind of metrafenone of the present invention are as follows:
[0048] (1) Diazotization reaction:
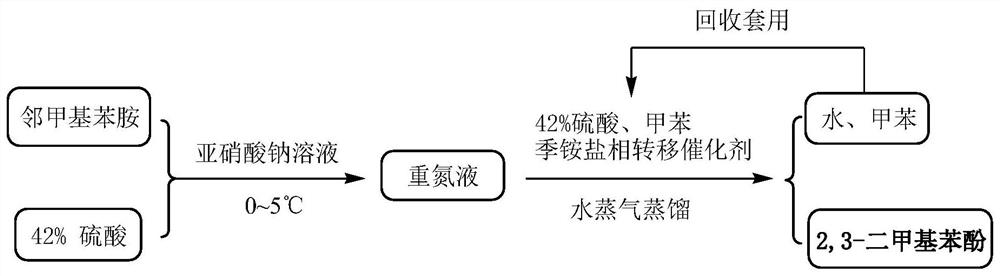
[0049] Depend on figure 1 As can be seen from the diazotization reaction process flow chart shown, the temperature of the mixed solution of 400kg of 42wt% sulfuric acid and 128.8kg of 2,3-dimethylaniline is lowered to -5~0°C, and then dropwise below the liquid surface Add 234.6kg of pre-prepared 30wt% sodium nitrite solution and stir well to obtain a diazo solution, which is stored below 0-5°C for direct use in the next step. Attention should be paid to the safety of diazotization in this step.
[0050] (2) Hydrolysis reaction:
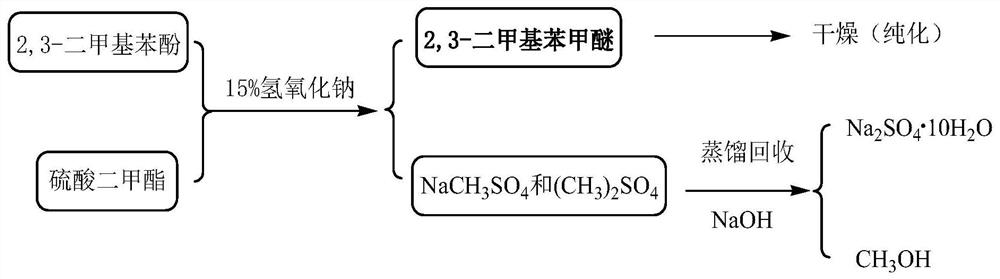
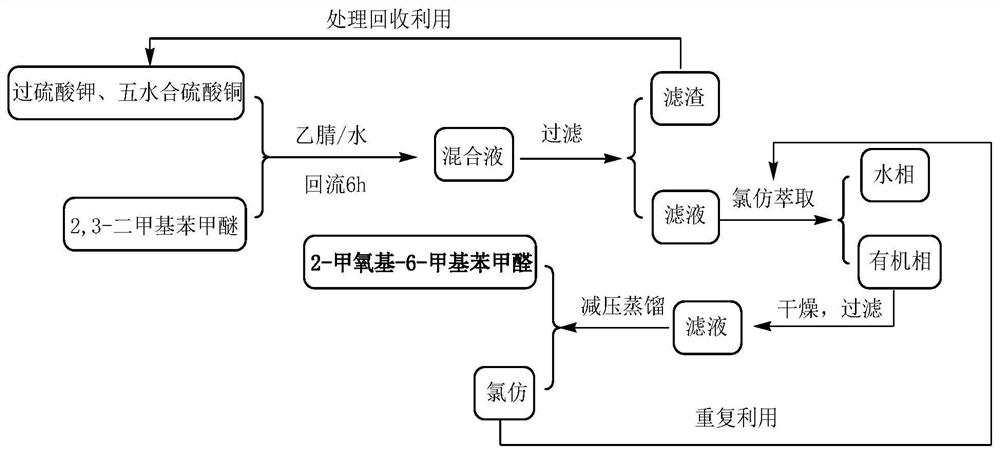
[0051] Depend on figure 1 Shown hydrolysis reaction process flow sheet finds out, to the diazonium liquid of step 1 gained, add the sulfuric acid 534kg of boiling 42wt%, toluene 272kg and 2mmol quaternary ammonium salt phase-transfer catalyst (0.68kg tetrabutyl ammonium bromide) mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com