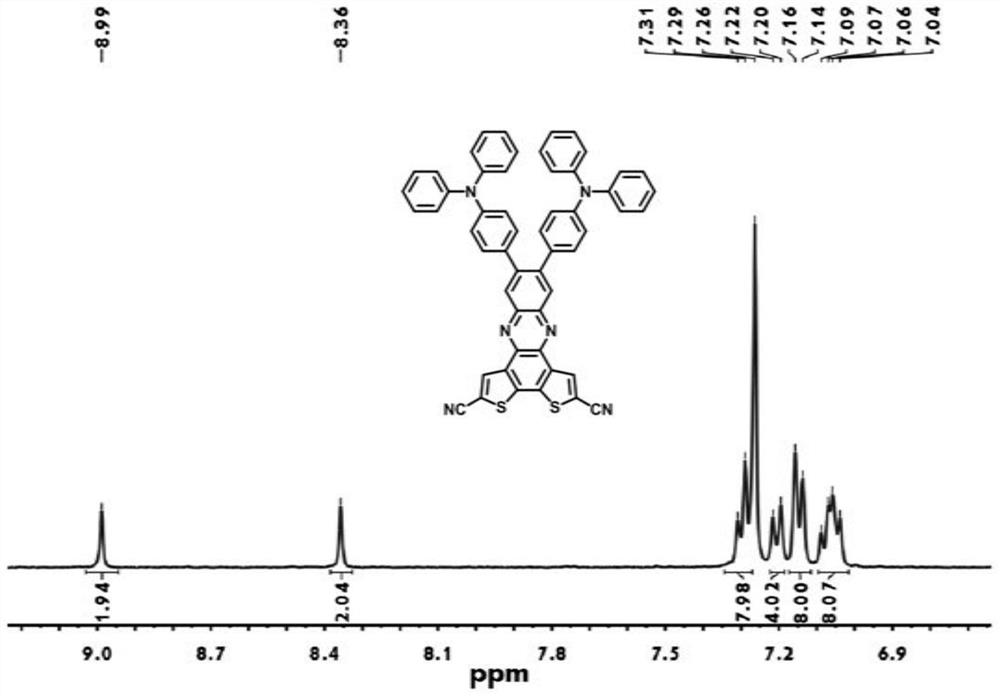
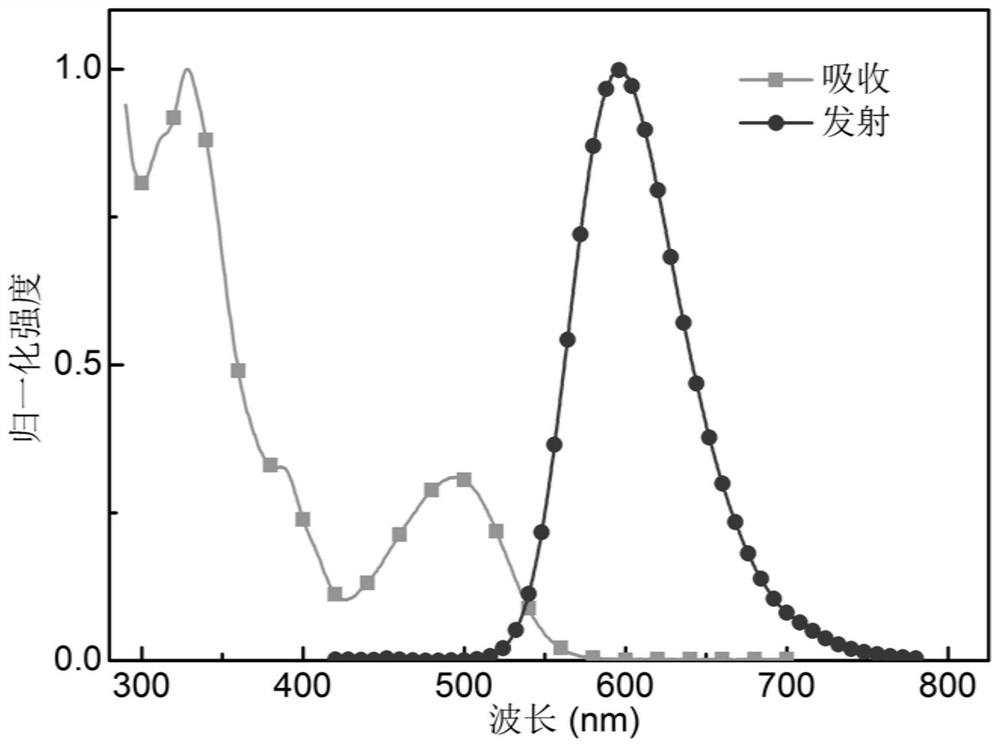
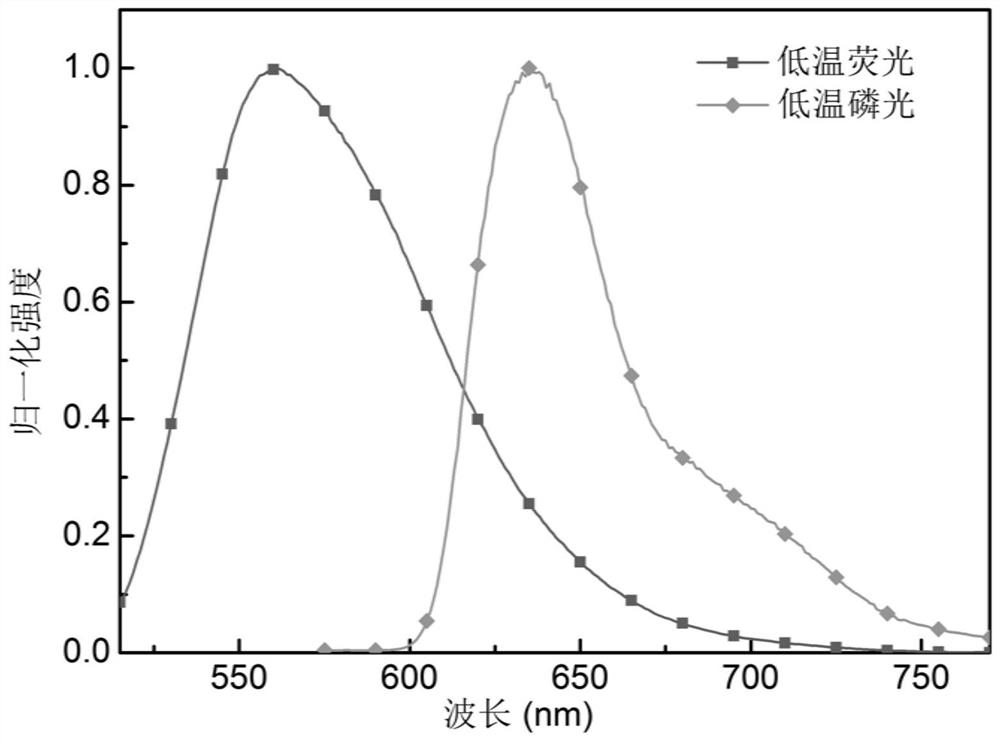
Organic electroluminescent compound based on phenazine-thiophene-dicyanogen and application
An organic light-emitting layer and luminescent technology, applied in organic chemistry, light-emitting materials, circuits, etc., to reduce production costs and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0065] Embodiment 3 organic electroluminescence device
[0066] The present invention also provides an organic electroluminescent device prepared by a thermally activated delayed fluorescent material based on the above-mentioned phenazine-thiophene-dicyano planar system, which consists of an anode 1, a hole injection layer 2, The hole transport layer 3, the electron blocking layer 4, the organic light-emitting layer 5, the electron transport layer 6 and the cathode 7 are composed.
[0067] Wherein, the organic light-emitting layer 5 is formed by doping the compound shown in formula 2-5 prepared in Example 1 of the present invention into the host material, and the doping ratios are 10wt.%, 15wt.% and 20wt.%. "Doping ratio" refers to the mass fraction of the compound represented by formula 2-5 in the total weight of the organic light-emitting layer.
[0068] The manufacturing method of the above organic electroluminescent device is prepared by a conventional method. Specifical...
Embodiment 4
[0079] This embodiment provides an organic electroluminescent device, the structure of which is the same as that in Embodiment 3, except that the organic light-emitting layer 5 is doped with the compound shown in Formula 2-6 prepared in Embodiment 2 of the present invention Formed in the host material, its doping ratio is 5wt.%, 10wt.% and 15wt.%.
[0080] The three organic electroluminescent devices OLED4-OLED6 provided in this example use CBP as the host material. The materials and thicknesses of the prepared devices are as follows:
[0081] OLED4: ITO / HAT-CN (10nm) / TAPC (40nm) / TCTA (10nm) / CBP:5wt% compound (20nm) / B4PyMPM (50nm) / Liq (2nm) / Al (120nm) shown in formula 2-6 );
[0082] OLED5: ITO / HAT-CN (10nm) / TAPC (40nm) / TCTA (10nm) / CBP:10wt% compound shown in formula 2-6 (20nm) / B4PyMPM (50nm) / Liq (2nm) / Al (120nm );
[0083] OLED6: ITO / HAT-CN(10nm) / TAPC(40nm) / TCTA(10nm) / CBP:15wt% compound (20nm) / B4PyMPM(50nm) / Liq(2nm) / Al(120nm) shown in formula 2-6 );
[0084] The structu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum external quantum efficiency | aaaaa | aaaaa |
| Maximum external quantum efficiency | aaaaa | aaaaa |
| Maximum external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com