Application of CTLA4 gene and PD1 gene humanized mouse model
A mouse model, humanized technology, applied in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of complex operation, unstable results, and high experimental costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides a method for constructing a CTLA4 gene and PD1 gene humanized BALB / c mouse model, which includes the following model construction method:
[0041] According to the method of patent CN 109266656 A, a humanized mouse model of PD1 gene modification was constructed, and then according to the method of patent CN 109022443 A, an sgRNA expressing the CTLA4 gene of BALB / c mice was constructed, and then according to the obtained sgRNA, a human source of CTLA4 was constructed. sequence carrier, and then inject the sgRNA against the BALB / c mouse CTLA4 gene and the carrier carrying the CTLA4 human sequence, as well as Cas9 mRNA or Cas9 protein, into the fertilized egg cells of the above-mentioned (patent CN 109266656 A) PD1 gene-modified humanized mouse Humanized mouse model in which the PD1 gene and CTLA4 gene are simultaneously modified by transplantation into the recipient mother mouse in the cytoplasm or nucleus. The obtained mouse model is named BALB / c...
Embodiment 2
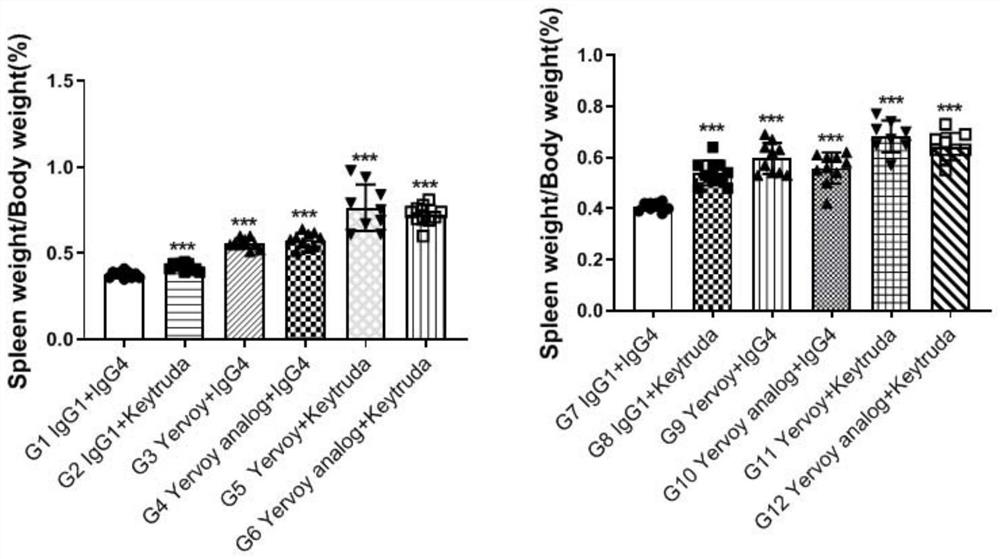
[0045] BALB / c-hPD1 / hCTLA4 adult mice constructed in Example 1 were used for toxicological evaluation test, 120 of them were 5-6 weeks old, female. All data were plotted by GraphPad Prism. The data between the two groups were analyzed by unpaired two-tailed Student's t-test. The supplier of Yervoy analog is BIOINTRON, the supplier of Keytruda is MSD Ireland, and the supplier of Yervoy is Bristol-Myers Squibb.
[0046] BALB / c-hPD1 / hCTLA4 mice aged 5-6 weeks were treated with Keytruda antibody, Yervoy antibody, Yervoy analog alone, or in combination with Keytruda antibody and Yervoy antibody, or in combination with Keytruda antibody and Yervoyanalog. By intraperitoneal injection (i.p.), once every 3 days, the administration frequency is shown in Table 1. On the 9th or 21st day after administration, the mice were euthanized, serum, tissues and organs were collected, and toxicology-related indicators were tested.
[0047] IgG 1 and IgG 4 are irrelevant antibodies, used for blank...
Embodiment 3
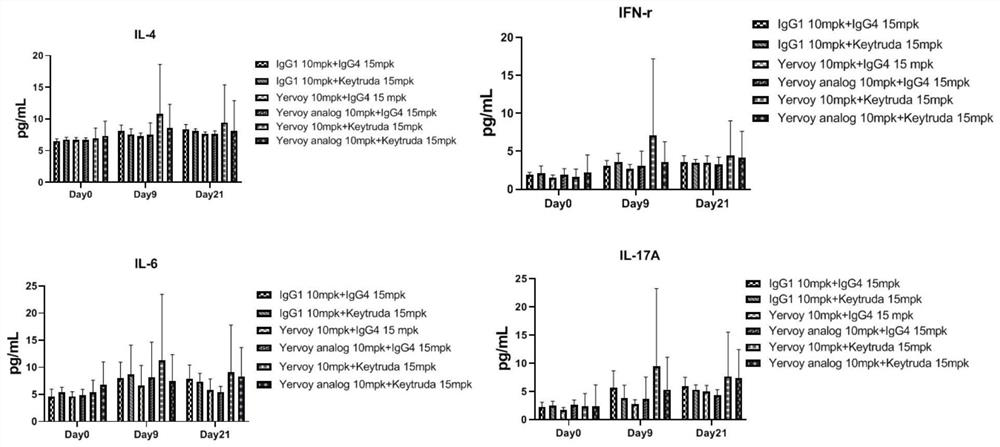
[0051] This example shows the expression of inflammatory cytokines in plasma of model mice after administration. Group 1 (G1) to Group 6 (G6) received orbital blood collection at D0 and D9; groups G7-G12 received orbital blood collection at D0 and D21, and separated blood plasma using CBA (Cytometric Bead Array) kit (BD, 560485) ) for cytokine detection.
[0052] Cytokine test results refer to figure 1 As shown, compared with the control group, on the 9th and 21st day, the inflammatory factors IL-4, IL-6, IFN-r , the expression of IL-17A increased, indicating that after the combined treatment of PD1 / CTLA4 antibody, the mice showed an immune-related inflammatory response, which was similar to the immune-related side effect phenotype caused by clinical hPD1 / hCTLA4 antibody drug treatment in patients .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com