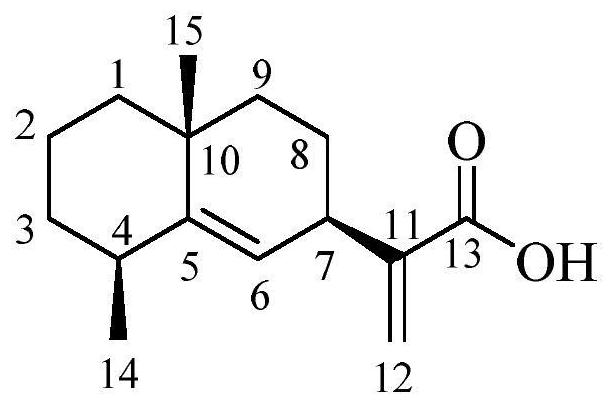
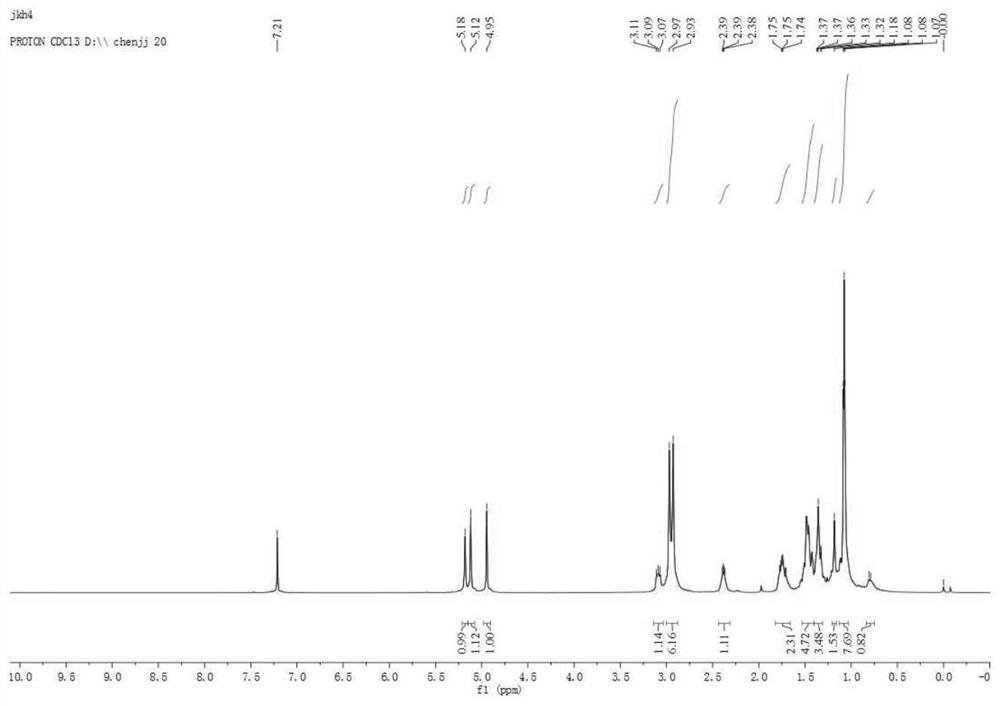
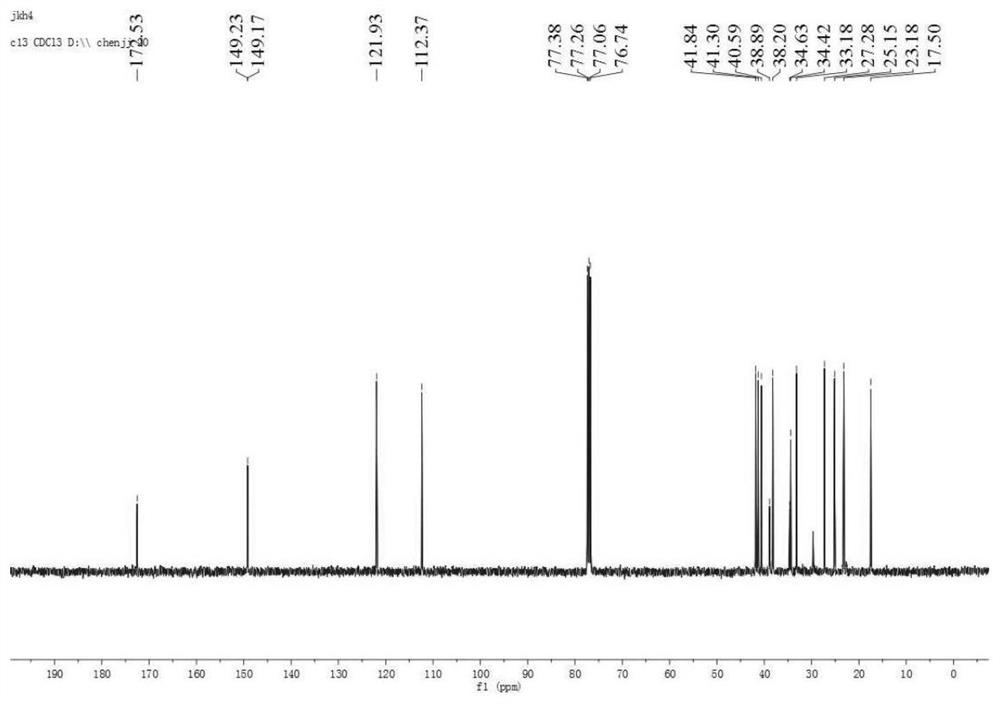
Pterodontic acid derivatives as well as preparation method and application thereof
A technology of malodotanic acid and its derivatives, applied in the field of natural medicinal chemistry, can solve the problems of large toxic side effects and easy complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The synthesis of the 13 amidation derivatives of embodiment 1 malidan acid
[0090] Reaction (i) is carried out in the present embodiment: 50.0 mg of fendanic acid with the structure of formula (A) is dissolved in 5.0 mL of anhydrous dichloromethane (DCM) solvent, and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI, 0.27mmol) as condensation agent, 4-dimethylaminopyridine (DMAP) catalyst (0.1mmol), add 0.25mmol methylamine hydrochloride, ethylamine hydrochloride salt, allylamine, dimethylamine hydrochloride, diethylamine hydrochloride, diallylamine, 2,4-dimethylhexylamine, cyclohexylamine, 2-methyl-2 imidazoline, 4-picolylamine, 4-methoxybenzylamine, 3-aminobenzylamine, methylbenzylamine, 3-chlorobenzylamine, 4-chlorobenzylamine, 4-fluorobenzylamine, 4-bromobenzylamine, 4-Hydroxybenzylamine, diphenylmethylamine, benzylamine, 4-methylbenzylamine, phenethylamine, 2-furylmethylamine, 4-(trifluoromethyl)benzylamine, 3-( Trifluoromethyl)benzylamine, 2-chl...
Embodiment 2
[0091] Synthesis of the 13-position amidation derivatives of embodiment 2 malidanic acid
[0092] Reaction (i) is carried out in the present embodiment: 80.0 mg of fendanic acid of formula (A) structure is dissolved in 8.0 mL of anhydrous dichloromethane (DCM) solvent, and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI, 0.43mmol) as condensation agent, 4-dimethylaminopyridine (DMAP) catalyst (0.2mmol), add 0.40mmol methylamine hydrochloride, ethylamine hydrochloride salt, allylamine, dimethylamine hydrochloride, diethylamine hydrochloride, diallylamine, 2,4-dimethylhexylamine, cyclohexylamine, 2-methyl-2 imidazoline, 4-picolylamine, 4-methoxybenzylamine, 3-aminobenzylamine, methylbenzylamine, 3-chlorobenzylamine, 4-chlorobenzylamine, 4-fluorobenzylamine, 4-bromobenzylamine, 4-Hydroxybenzylamine, diphenylmethylamine, benzylamine, 4-methylbenzylamine, phenethylamine, 2-furylmethylamine, 4-(trifluoromethyl)benzylamine, 3-( Trifluoromethyl)benzylamine, 2-chlor...
Embodiment 3
[0093] Synthesis of the 13 amidation derivatives of embodiment 3 malidanic acid
[0094] Reaction (i) is carried out in the present embodiment: 60.0 mg of meridanic acid of formula (A) structure is dissolved in 6.0 mL of anhydrous dichloromethane (DCM) solvent, and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCI, 0.3mmol) as condensation agent, 4-dimethylaminopyridine (DMAP) catalyst (0.15mmol), add 0.35mmol methylamine hydrochloride, ethylamine hydrochloride salt, allylamine, dimethylamine hydrochloride, diethylamine hydrochloride, diallylamine, 2,4-dimethylhexylamine, cyclohexylamine, 2-methyl-2 imidazoline, 4-picolylamine, 4-methoxybenzylamine, 3-aminobenzylamine, methylbenzylamine, 3-chlorobenzylamine, 4-chlorobenzylamine, 4-fluorobenzylamine, 4-bromobenzylamine, 4-Hydroxybenzylamine, diphenylmethylamine, benzylamine, 4-methylbenzylamine, phenethylamine, 2-furylmethylamine, 4-(trifluoromethyl)benzylamine, 3-( Trifluoromethyl)benzylamine, 2-chlorobenzyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com