Pharmaceutical composition for treating African swine fever and application thereof
A technology of African swine fever and a composition, applied in the field of medicine, can solve the problems of reduced enzyme protein activity, interfere with calcium operation, functional disorders, etc., and achieve the effects of improving inflammatory response, shortening the course of disease, and improving the level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
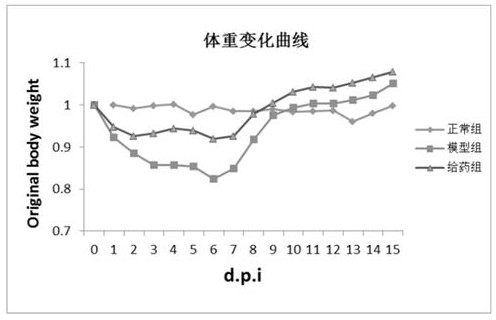
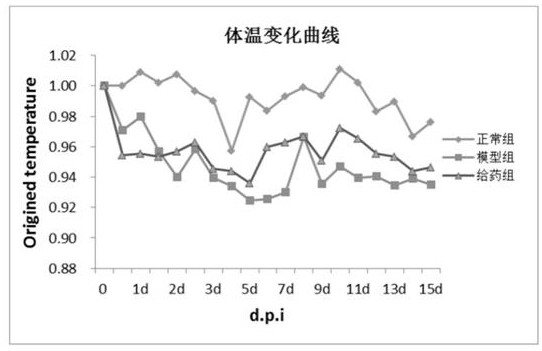
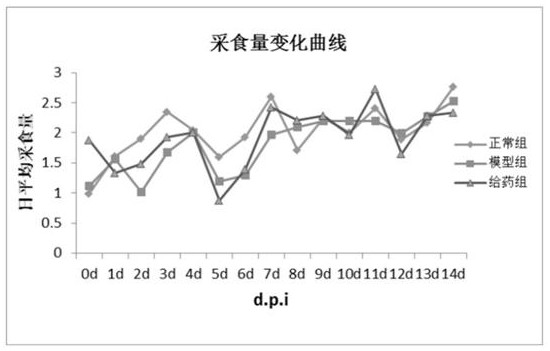
Image
Examples
Embodiment Construction
[0067] NO-induced stabilization of HIF-1α and phosphorylated p53 levels reduced by reactive oxygen species [Thomas DD, RidnourLA, Espey MG, et al. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281(36) :25984–25993.], and indeed, the addition of antioxidants has a protective effect on nitrosative signaling [Edirisinghe I, Arunachalam G, Wong C, et al. Cigarette-smoke-inducedoxidative / nitrosative stress impairs VEGF- and fluid-shear-stress -mediated signaling in endothelial cells [retracted in: Rahman I. Antioxid RedoxSignal. 2013 Apr 2018(12):1535]. Antioxid Redox Signal. 2010;12(12):1355–1369.], thus NO levels and subsequent downstream signaling Conduction is regulated by reactive oxygen species and is also a factor in modulating redox signaling.
[0068] The expression of NOS requires the simultaneous activation of STAT and NF-κB, and NF-κB acts as the master switch of inflammation, and H 2 o 2 Production-related, NF-κB is regulated by redox. Mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com