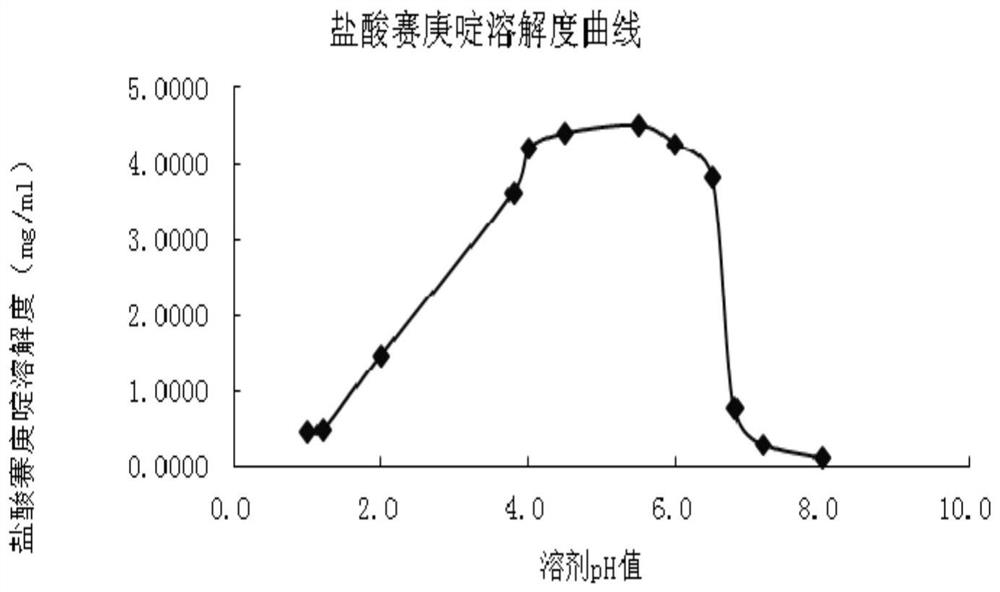
Cyproheptadine hydrochloride quick-release pharmaceutical preparation and preparation method thereof
A technology for cyproheptadine hydrochloride and pharmaceutical preparations, which is applied in the field of rapid-release pharmaceutical preparations and preparations of cyproheptadine hydrochloride, which can solve the problems of poor drug absorption and achieve high blood drug concentration, high solubility, and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a kind of preparation method of cyproheptadine hydrochloride fast-release pharmaceutical preparation, comprising the steps of:
[0043] (1) Drying each component of the pH control layer and then granulating and mixing uniformly;
[0044] (2) Mix the components of the active layer after sieving, dry and mix uniformly after wet granulation;
[0045] (3) The pH control layer and the active layer obtained in step (1) and step (2) are jointly compressed into a preparation structure in which the inner layer is an active layer with cyproheptadine hydrochloride as an active ingredient and the outer layer is a pH control layer, That is, the rapid release pharmaceutical preparation of cyproheptadine hydrochloride is obtained.
[0046] In some embodiments, step (1) specifically includes the following steps: first, each component of the control layer is dried under suitable temperature conditions, for example, mannitol, disodium hydrogen phospha...
specific Embodiment
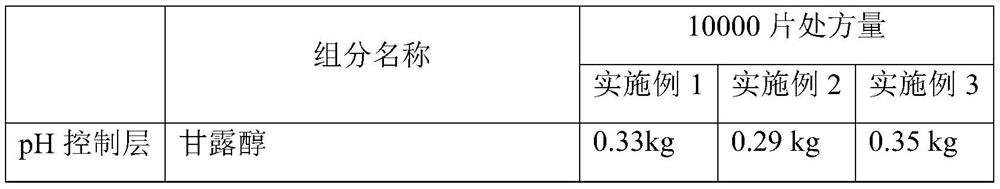
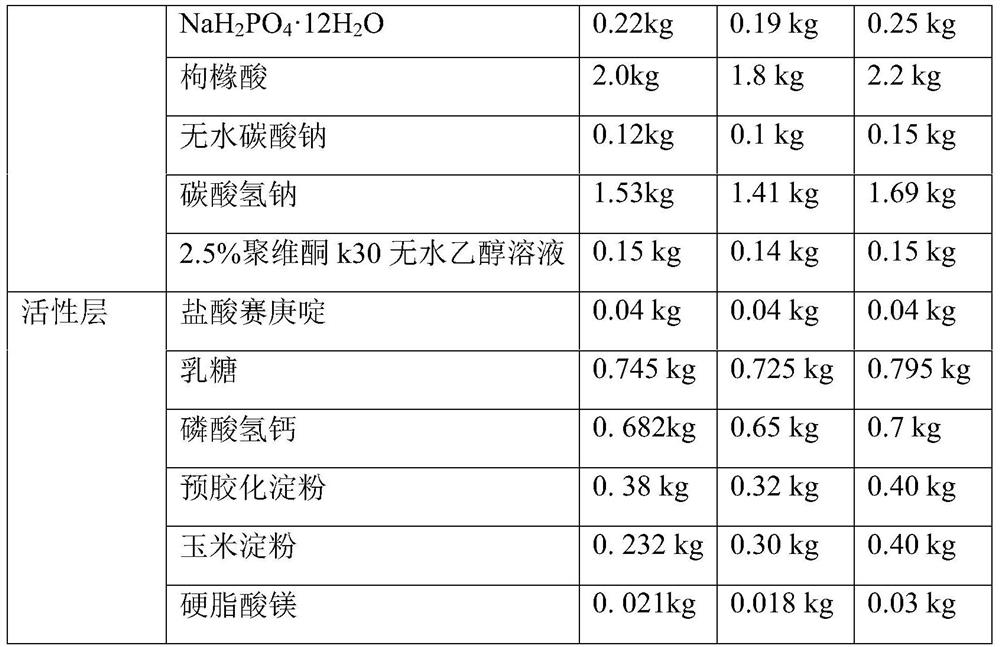
[0052] The rapid-release pharmaceutical preparation of cyproheptadine hydrochloride provided in this example comprises an inner active layer with cyproheptadine hydrochloride as the main active ingredient and an outer pH control layer. Wherein the outer pH control layer (also known as the effervescent layer) and the active layer mainly include ingredients and content as shown in Table 1:
[0053] Table 1 Example 1 to Example 3 Cyproheptadine hydrochloride quick-release pharmaceutical preparations, its inner layer active layer and outer layer pH control layer components and the corresponding prescription quantity of 10000 tablets of this preparation
[0054]
[0055]
[0056] The pharmaceutical preparation is an effervescent tablet, and the pH control layer granules of the effervescent tablet are prepared according to the following preparation method:
[0057] (1-1) Mannitol, NaH 2 PO 4 12H 2 O, citric acid, anhydrous sodium carbonate, sodium bicarbonate were dried at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com