Method for catalyzing ORR reaction based on COFs derived carbon nanotubes
A carbon nanotube and reaction technology, applied in the field of electrocatalytic oxygen reduction in the cathode part of fuel cells, can solve the problems of low Pt reserves and high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
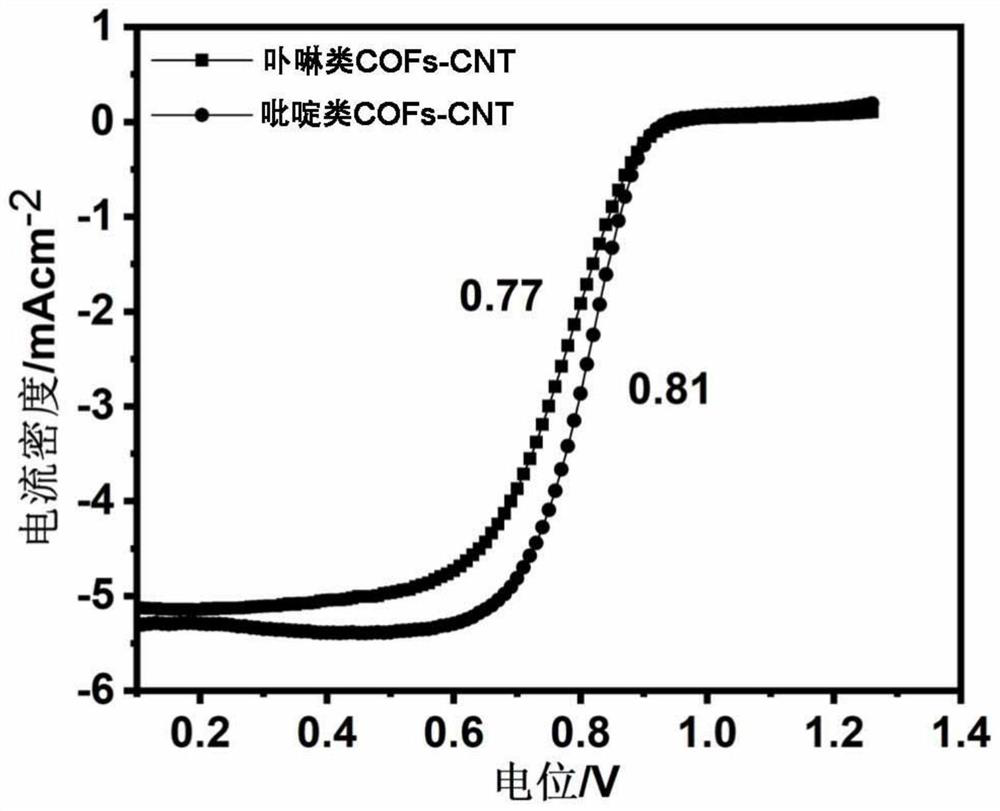
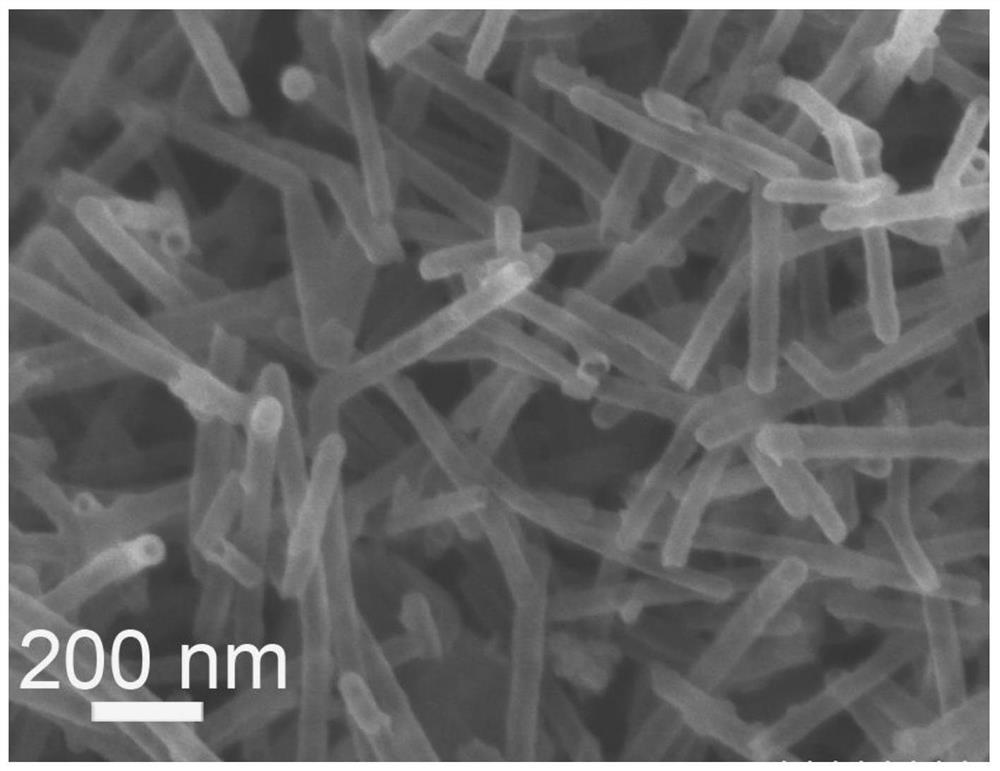
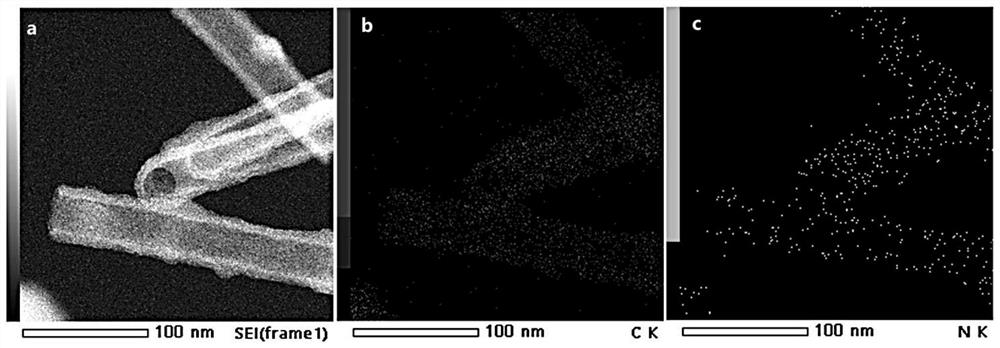
[0081] The application of carbon nanotubes derived from covalent organic frameworks (COFs) in the catalytic cathode oxygen reduction reaction includes the following steps: using carbon nanotubes derived from covalent organic frameworks (COFs) as a catalyst in the cathode oxygen reduction reaction; specifically: weighing 0.5 mg of carbon nanotube solid powder derived from covalent organic frameworks COFs was added to 80 microliters of isopropanol aqueous solution (volume ratio of isopropanol to water=3:1) and 5 microliters of perfluorosulfonic acid Nafion solution (mass fraction 5 %) in the mixed solution, ultrasonically uniform; then drop 10 microliters on an area of 0.192cm 2 The working electrode is obtained on the glassy carbon electrode; finally, the working electrode is applied to catalyze the oxygen evolution reaction in electrolyzed water.
[0082] The specific catalytic cathode oxygen reduction reaction test is to place the working electrode in a 0.1M potassium hydro...
Embodiment 2
[0088] An application of carbon nanotubes derived from covalent organic frameworks (COFs) in catalyzing cathode oxygen reduction reactions, comprising the following steps: using carbon nanotubes derived from covalent organic frameworks (COFs) as a catalyst in cathode oxygen reduction reactions; specifically: Weigh 0.5 mg of carbon nanotube solid powder derived from covalent organic frameworks COFs, add 80 microliters of isopropanol aqueous solution (volume ratio of isopropanol to water=3:1) and 5 microliters of perfluorosulfonic acid Nafion solution (mass Fraction 5%) in the mixed solution, ultrasonically uniform; then drop 10 microliters on an area of 0.192cm 2 The working electrode is obtained on the glassy carbon electrode; finally, the working electrode is applied to catalyze the oxygen evolution reaction in electrolyzed water. The specific catalytic cathode oxygen reduction reaction test is to place the working electrode in a 0.1M potassium hydroxide solution to test it...
Embodiment 3
[0094] An application of carbon nanotubes derived from covalent organic frameworks (COFs) in catalyzing cathode oxygen reduction reactions, comprising the following steps: using carbon nanotubes derived from covalent organic frameworks (COFs) as a catalyst in cathode oxygen reduction reactions; specifically: Weigh 0.5 mg of carbon nanotube solid powder derived from covalent organic frameworks COFs, add 80 microliters of isopropanol aqueous solution (volume ratio of isopropanol to water=3:1) and 5 microliters of perfluorosulfonic acid Nafion solution (mass Fraction 5%) in the mixed solution, ultrasonically uniform; then drop 10 microliters on an area of 0.192cm 2 The working electrode is obtained on the glassy carbon electrode; finally, the working electrode is applied to catalyze the oxygen evolution reaction in electrolyzed water. The specific catalytic cathode oxygen reduction reaction test is to place the working electrode in a 0.1M potassium hydroxide solution to test it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| Limiting current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com