A nickel-cobalt binary catalyst promoting the direct oxidation of sodium borohydride
A sodium borohydride and catalyst technology, applied in the field of electrochemical applications, can solve problems such as loss of catalytic activity, slow reaction rate, and increased anode open circuit potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
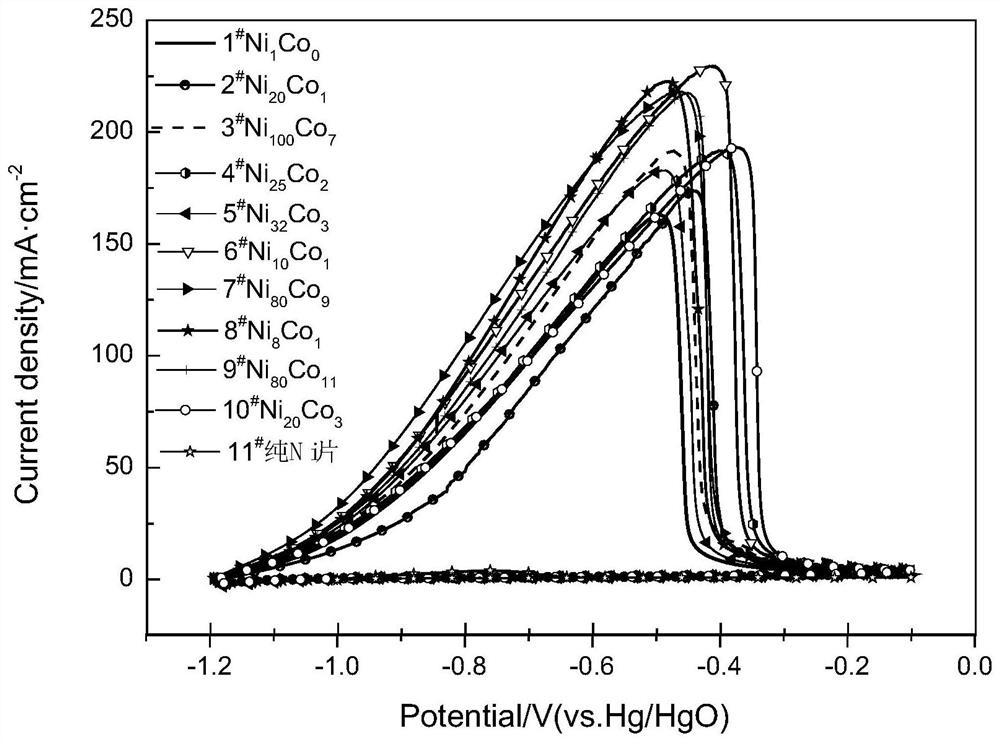
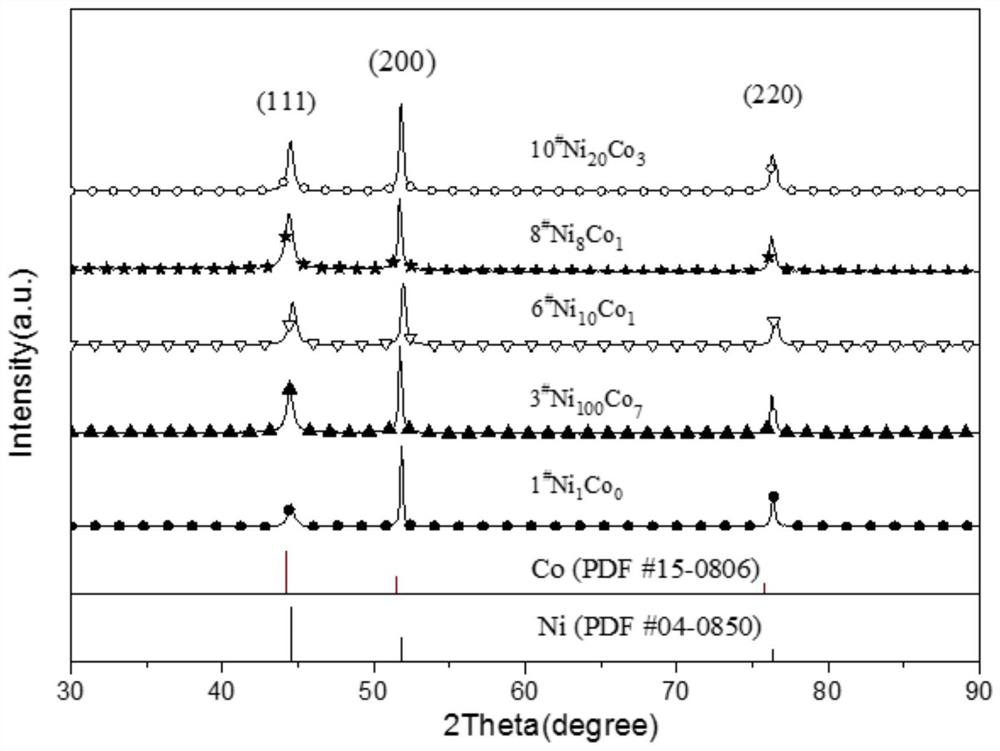
[0021] Under normal pressure, at a room temperature between 20 and 30°C, prepare 10 groups of nickel chloride (NiCl 2 ·6H 2 O), cobalt chloride (CoCl 2 ·6H 2 O) and ammonium chloride (NH 4 Cl) 100mL mixed solution, as electrodeposition solution, wherein the fixed nickel chloride concentration is 0.2mol / dm 3 , the concentration of ammonium chloride is 4mol / dm 3 , the concentrations of cobalt chloride are 0.0000, 0.0100, 0.0140, 0.0160, 0.01875, 0.0200, 0.0225, 0.0250, 0.0275, 0.0300mol / dm 3 , preparation of electrodeposition solution. Put a polished and smooth Ni sheet of 1cm×2cm as the cathode in the above solution, wherein the working area of the Ni sheet is 1cm×1cm, and the carbon rod is used as the anode, in a constant temperature water bath at 298.15K, using a constant current method, and the deposition current is 2A, the deposition time is 20s, and Ni and Co are deposited on the above-mentioned polished and smooth nickel sheet. When the concentration of cobalt ch...
Embodiment 2
[0026] Under normal pressure, the room temperature is between 20 and 30°C, and five groups of nickel chloride (NiCl 2 ·6H 2 O), cobalt chloride (CoCl 2 ·6H 2 O) and ammonium chloride (NH 4 Cl) 100mL mixed solution, as electrodeposition solution, wherein the fixed nickel chloride concentration is 0.2mol / dm 3 , the concentration of ammonium chloride is 4mol / dm 3 , the concentrations of cobalt chloride are 0.0000, 0.0140, 0.0200, 0.0250, 0.0300mol / dm 3 , preparation of electrodeposition solution. Put a polished and smooth Ni sheet of 1cm×2 cm as the cathode in the above solution. The working area of the Ni sheet is 1cm×1 cm. The carbon rod is used as the anode, and the constant current method is used in a constant temperature water bath at 298.15K. The deposition current was 2A, the deposition time was 20s, and Ni and Co were deposited on the above-mentioned polished and smooth nickel sheet. When the concentration of cobalt chloride is 0.0000, 0.0140, 0.0200, 0.0250, 0.0...
Embodiment 3
[0033] Under normal pressure, the room temperature is between 20 and 30°C, and five groups of nickel chloride (NiCl 2 ·6H 2 O), cobalt chloride (CoCl 2 ·6H 2 O) and ammonium chloride (NH 4 Cl) 100mL mixed solution, as electrodeposition solution, wherein the fixed nickel chloride concentration is 0.2mol / dm 3 , the concentration of ammonium chloride is 4mol / dm 3 , the concentrations of cobalt chloride are 0.0000, 0.0140, 0.0200, 0.0250, 0.0300mol / dm 3 , preparation of electrodeposition solution. A polished and smooth Ni sheet of 1 cm × 2 cm was placed in the above solution as the cathode, where the working area of the Ni sheet was 1 cm × 1 cm, and a carbon rod was used as the anode. In a constant temperature water bath at 298.15K, the constant current method was used to deposit The current is 2A, the deposition time is 20s, and Ni and Co are deposited on the above-mentioned polished and smooth nickel sheet. When the concentration of cobalt chloride is 0.0000, 0.0140, 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com