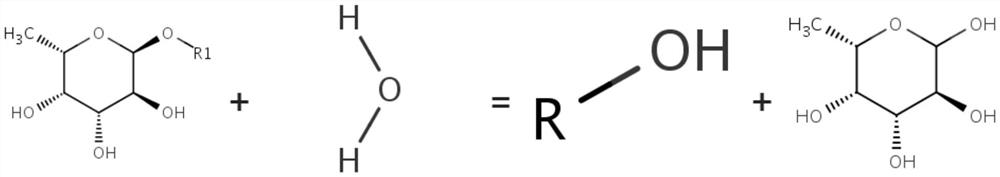Preparation method of alpha-L-fucosidase
A fucosidase and amino acid technology, applied in the fields of biochemistry, molecular biology, and genetic engineering, can solve the problems of unstable source, poor repeatability, and high price, and achieve high repeatability, high stability, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, design and construction of recombinant vector
[0037] The species source of the α-L-fucosidase in this example is: Fibrella aestuarina BUZ 2, and the Uniprot number is: IOK5U2. The amino acid sequence of the α-L-fucosidase in this embodiment is shown in SEQ ID No.1, and the nucleotide sequence of the gene encoding the α-L-fucosidase is shown in SEQ ID No.2.
[0038] In this example, the part of the amino-terminal signal peptide sequence was removed by artificially synthesizing the full-length gene sequence, and it was connected into the vector pET26b. The pET series vectors (formerly Novagen, now Merck Millipore) used in the present invention have enzyme cleavage sites of NdeI and XhoI . The gene synthesis and vector construction were completed in Sangon Bioengineering (Shanghai) Co., Ltd., and the constructed recombinant vector was named pSF14.
[0039] Transform the plasmid vector pSF14 into Escherichia coli BL21 (DE3) competent cells by chemical meth...
Embodiment 2
[0041] Embodiment 2, expression of α-L-fucosidase
[0042] Pick the strain expressing α-L-fucosidase obtained from the positive transformant, inoculate the recombinant expression strain in 5 ml liquid LB medium, and culture with shaking at 37° C. and 250 rpm for 7-8 hours.
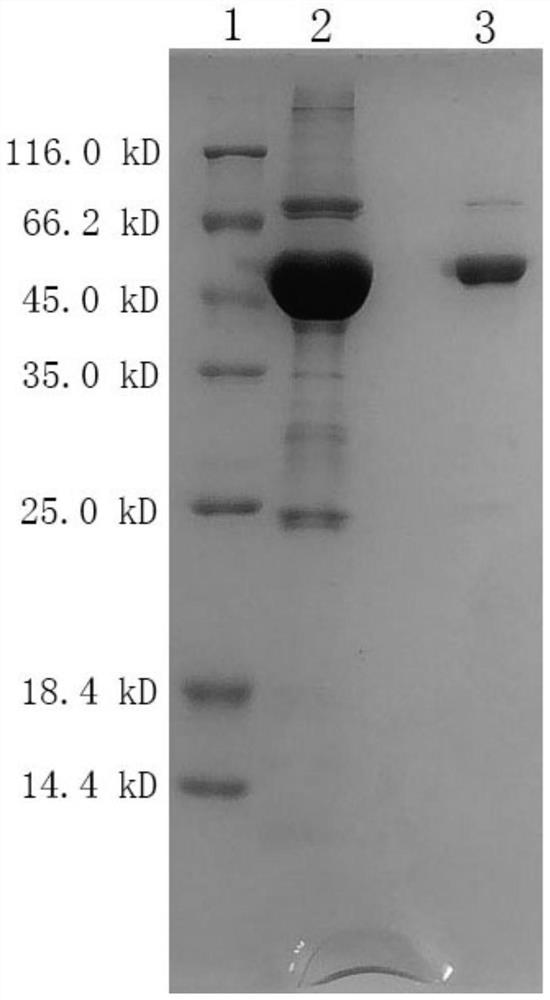
[0043] Transfer the strain culture solution to 100ml LB liquid medium, shake and cultivate overnight at 37°C and 250rpm; the next day, transfer the strain culture solution to two 3L culture flasks, each containing 1L LB medium, 37 Cultivate with shaking at 250 rpm until the absorbance at 600 nm is 0.8-1.0. The inducer IPTG was added to a final concentration of 0.1 mM, and the culture was shaken at 16° C. and 180 rpm for 24 hours. The bacterial cells were collected by centrifugation at 8000 rpm for 10 minutes. The bacterial pellet was resuspended with 20mM Tris-HCl pH8.0 and 200mM NaCl, and ultrasonically disrupted for 40min. After the lysate was centrifuged at high speed at 14000 rpm for 30 min, the sup...
Embodiment 3
[0044] The determination of embodiment 3α-L-fucosidase activity
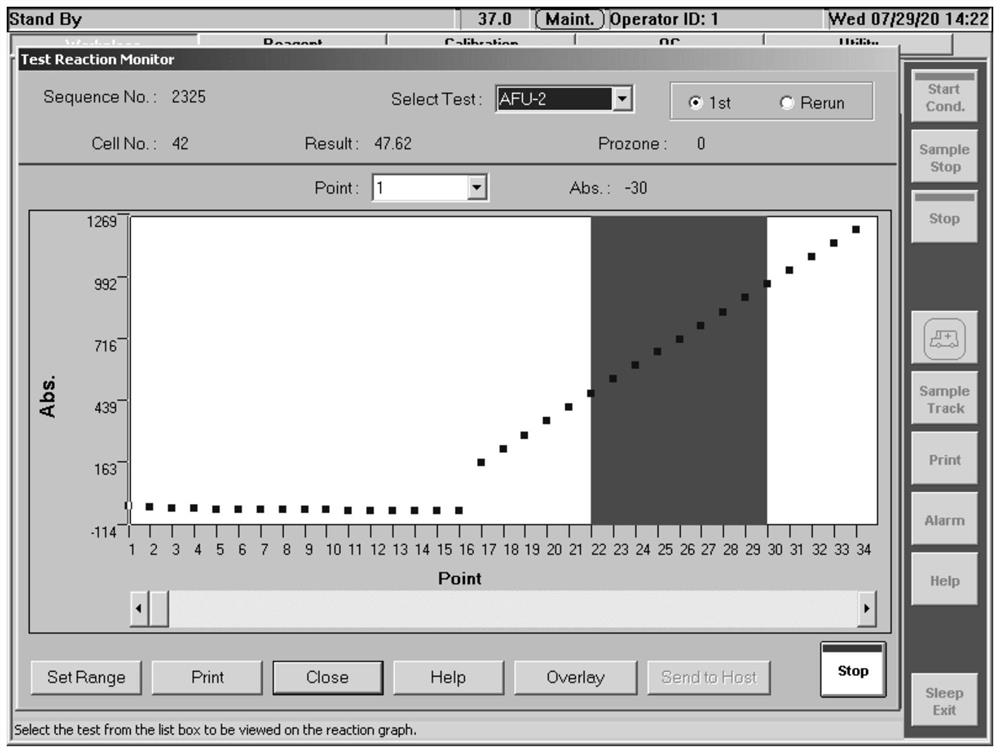
[0045] The α-L-fucosidase prepared in Example 2 is stored in the storage solution of α-L-fucosidase, and the formula of the storage solution is: 20mM Na 2 HPO 4 , 500mM NaCl, 10mM EDTA, pH6.5, take out a total of 10ml of α-L-fucosidase finished product, dilute 100 times, and use the self-developed kit of Haifeng Bio (Beijing) Technology Co., Ltd. and Hitachi 7180 biochemical analyzer to detect the enzyme Activity, the measured value is 72.99U / L, and the enzyme activity concentration of the finished product of α-L-fucosidase is 7.3U / ml.
[0046] Dilute the finished product of α-L-fucosidase 4 times, measure the absorbance at 280 nanometers with a Miou ultra-micro spectrophotometer, the measured value is 2.123, and the calculated protein concentration is 3.19mg / ml, the finished product of α-L-fucosidase The specific activity is 2.29U / mg.
[0047] After 100 times dilution of the finished α-L-fucosidase product, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com