Preparation method of 2-(5-bromo-3-methylpyridine-2-yl) acetic acid hydrochloride
A technology of acetic acid hydrochloride and picoline, applied in the direction of organic chemistry, etc., to achieve the effects of improving yield and purity, simple operation, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] A preparation method of 2-(5-bromo-3-methylpyridin-2-yl)acetic acid hydrochloride, the preparation method comprising the steps of:
[0020] (1) According to the volume ratio of n-butyllithium (2.5M), tetrahydrofuran and compound A in tetrahydrofuran solution is 16~18:100:35, the solid-liquid g / mL ratio of acetonitrile and compound A in tetrahydrofuran solution is 1:19 , take the raw material, put tetrahydrofuran into the reactor, protect it with nitrogen, add n-butyllithium (2.5M), cool down to -80~-75℃, add acetonitrile dropwise, keep warm for 1~2h, add tetrahydrofuran of compound A Solution, keep stirring for 2 hours, heat up to 30-35°C, and stir for 1 hour to obtain compound B;
[0021] (2) According to the solid-to-liquid g / mL ratio of compound B and concentrated hydrochloric acid (12M) as 1:4.5, take raw materials, mix compound B and concentrated hydrochloric acid (12M), raise the temperature to 80-85°C, and stir for 1-2 hours , to obtain compound C, which is 2-(5...
Embodiment 1
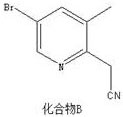
[0023] Preparation of Compound B:
[0024]
[0025] Put 1000mL tetrahydrofuran into the reactor, under nitrogen protection, add 160mL n-butyllithium (2.5M), cool down to -80°C, add 18.4mL acetonitrile dropwise, keep the reaction for 1h, add 350mL tetrahydrofuran solution containing 50g compound A, keep warm Stir for 2 hours, raise the temperature to 30°C, stir for 1 hour, add saturated aqueous ammonium chloride solution (500mL), extract with EA (500mL*3), combine the organic phases, mix the sample and pass through the column to obtain 35.3g of light yellow oil, and compound B, The yield was 83.9%, and the purity was 98.3%.
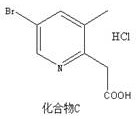
[0026] Compound C is the preparation of 2-(5-bromo-3-methylpyridin-2-yl)acetic acid hydrochloride:
[0027]
[0028] (2) Mix 30g of compound B and 135mL of concentrated hydrochloric acid (12M), raise the temperature to 80°C, stir and react for 1h, and detect by TLC. After the reaction of raw materials is completed, the solvent is distilled off under...
Embodiment 2
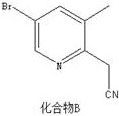
[0031] Preparation of Compound B:
[0032]
[0033] Put 1000mL tetrahydrofuran into the reactor, under nitrogen protection, add 170mL n-butyllithium (2.5M), cool down to -78°C, add 18.4mL acetonitrile dropwise, keep the reaction for 1.5h, add 350mL tetrahydrofuran solution containing 50g compound A, Keep stirring for 2 hours, raise the temperature to 33°C, stir for 1 hour, add saturated ammonium chloride aqueous solution (500mL), extract with EA (500mL*3), combine the organic phase, mix the sample and pass through the column to obtain 36.2 light yellow oil, and compound B, The yield was 86.0%, and the purity was 99.3%.
[0034] Compound C is the preparation of 2-(5-bromo-3-methylpyridin-2-yl)acetic acid hydrochloride:
[0035]
[0036] (2) Mix 30g of compound B and 135mL of concentrated hydrochloric acid (12M), raise the temperature to 83°C, stir and react for 1.5h, and detect by TLC. After the reaction of the raw materials is completed, the solvent is distilled off und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com