Rapid sterility inspection method
A rapid technology for sterility inspection, applied in the determination/inspection of microorganisms, biochemical equipment and methods, measuring devices, etc., can solve the problems of expensive equipment and reagents, interference from other particles, complicated operation, etc., and achieve short inspection time , easy operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0039] The comparison of the earliest observation time of embodiment 2
[0040] This embodiment is tested according to the method of Example 1, and detects the earliest observation time of different bacterial species by the traditional sterility test method (STD) and the rapid sterility test method (AMT) provided by the present invention.
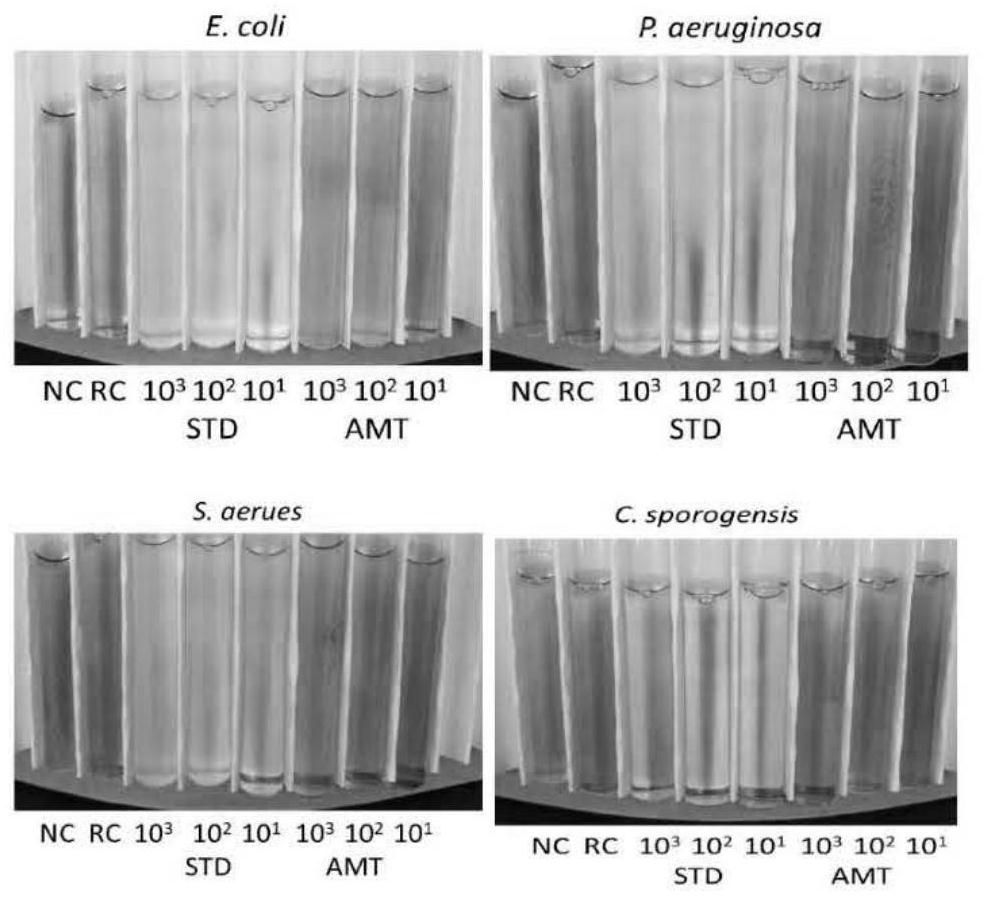
[0041] Among them, after cultivating for 8 hours, under the traditional method (STD) and this method (AMT) culture, different concentrations of Staphylococcus aureus (S.aerues), Escherichia coli (E.coli), Pseudomonas aeruginosa The color changes of P.aeruginosa and C.sporogensis are as follows: figure 1 shown. Depend on figure 1 It can be seen that, adopting the traditional method (STD) and the present method (AMT) after culturing for 8 hours, the color has no obvious change, and it is difficult to accurately distinguish whether there are bacteria with the naked eye.
[0042] A spectrometer was used to detect the different concentrations...
Embodiment 3
[0048] The comparison of embodiment 3 accuracy and limit of detection
[0049] This embodiment compares the accuracy and sensitivity of the traditional sterility testing method and the rapid sterility testing method provided by the present invention through experiments. Traditional sterility testing method wherein and the fast sterility testing method provided by the present invention are tested according to the method of embodiment 1, and two kinds of methods are tested in parallel 5 times respectively, traditional sterility testing method and the fast sterility testing method provided by the present invention The test results are shown in Table 3.
[0050] Table 3 traditional sterility testing method and rapid sterility testing method detection result comparison of the present invention
[0051]
[0052] As can be seen from Table 3, the inventive method has no difference with traditional pharmacopoeia method accuracy and detection limit.
[0053] Embodiment 4 method dur...
Embodiment 5
[0056] Embodiment 5 two kinds of indicator interaction test
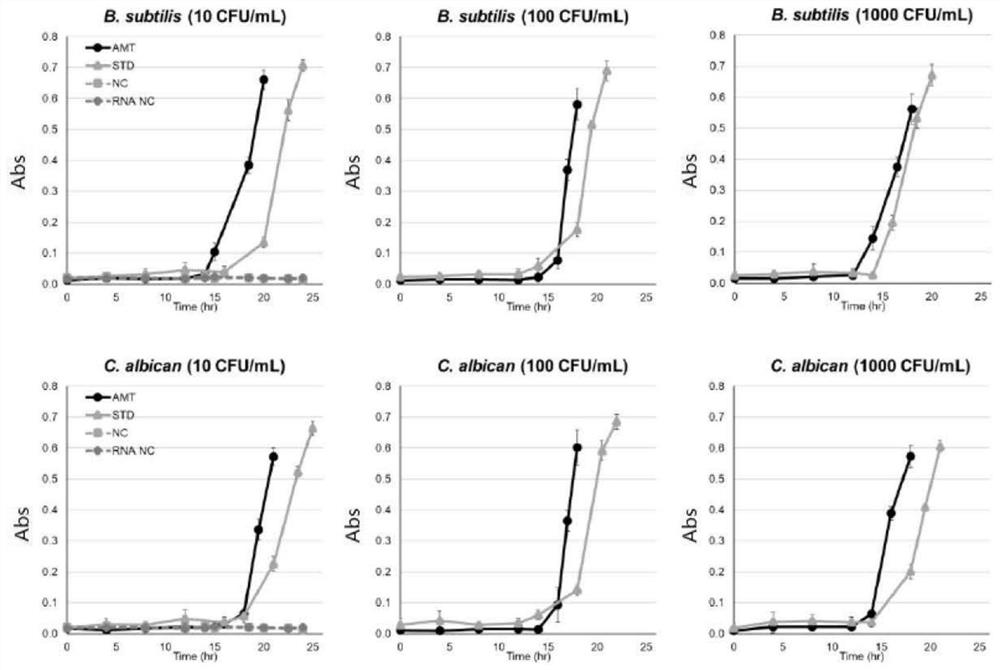
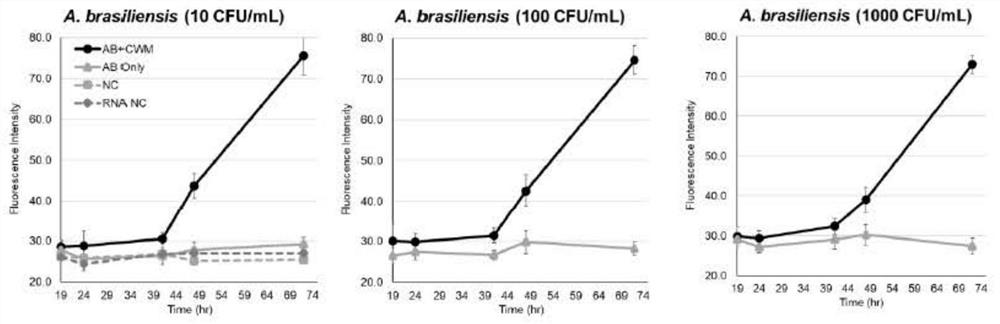
[0057] This embodiment adopts the rapid sterility testing method provided by the present invention according to Example 2, wherein the indicators are divided into three situations, the first one has only TTC, which is used to detect the large intestine with concentrations of 10, 100, and 1000 CFU / ml Bacteria, Pseudomonas aeruginosa, Staphylococcus aureus, Clostridium sporogenes, Bacillus subtilis and Candida albicans, investigate the earliest observation time (time point of first positive reaction); the second only CWM, used for Aspergillus niger with a detection concentration of 10, 100, and 1000 CFU / ml, to investigate the earliest observation time; the third type contains TTC+CWM, which is used to detect Escherichia coli and Pseudomonas aeruginosa at a concentration of 10, 100, and 1000 CFU / ml bacteria, Staphylococcus aureus, Clostridium sporogenes, Bacillus subtilis, Candida albicans and Aspergillus niger. Throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com