Bionic modified valve material as well as preparation method and application thereof
A biological valve and valve technology, applied in the field of biomedical materials, can solve the problems of valve thrombosis, calcified mechanical properties, and shortened valve life, and achieve the effects of enhanced drug release, wide sources, and mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
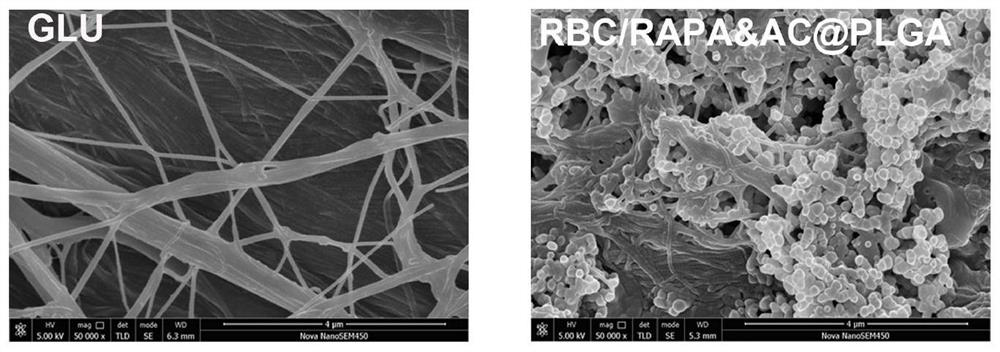
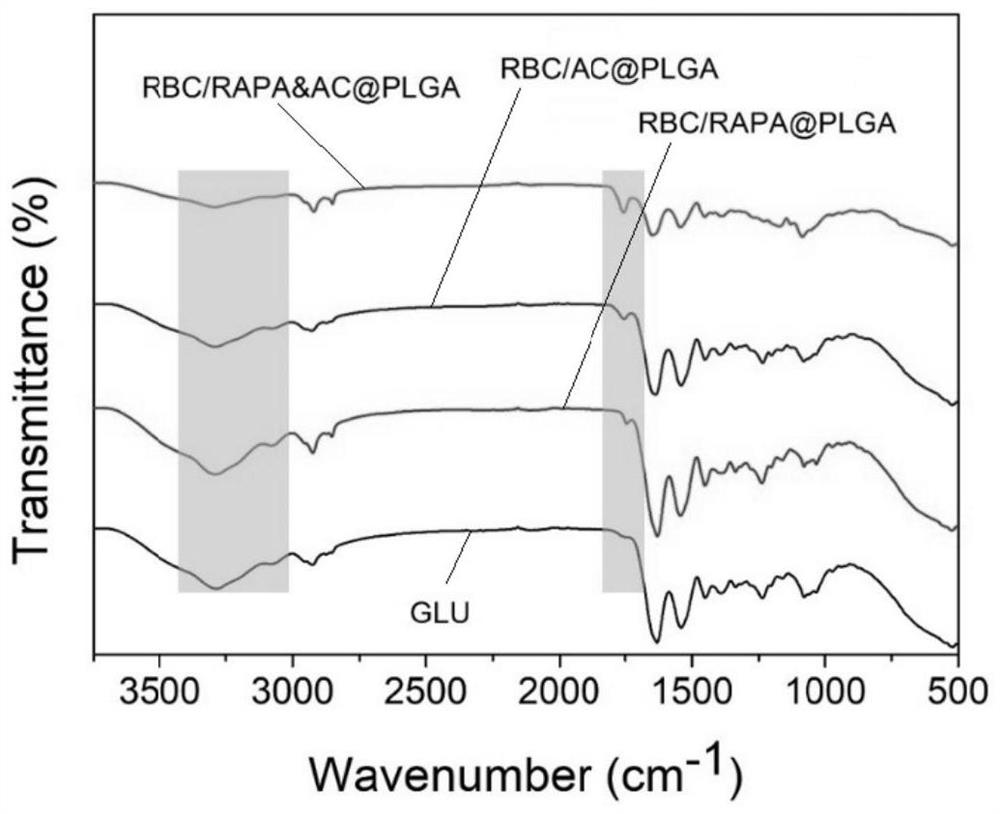
[0044] A preferred embodiment of the present invention provides a method for preparing a bionic modified valve material, specifically a method for preparing red blood cell membrane-wrapped nano drug-loaded particles for bionic modification of porcine pericardial valves, which is obtained by the following steps:
[0045] 1) Preparation of drug-loaded PLGA nanoparticles (RAPA&AC@PLGA)
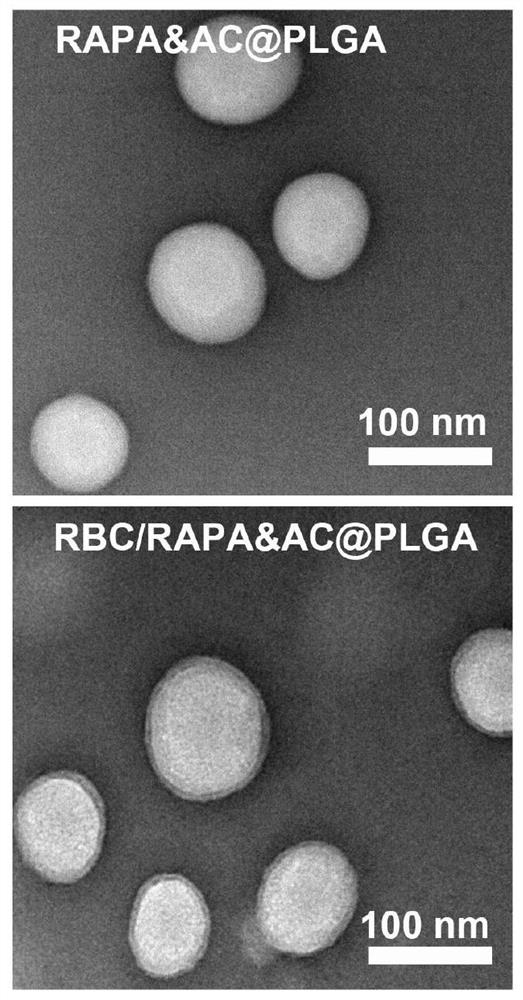
[0046] PLGA (10 mg, MW.50000, 50:50), RAPA (1 mg), AC (1 mg) were dissolved in 1 mL of dimethylsulfoxide (DMSO). Then the mixed solution was added dropwise to 3 mL of water and stirred gently. After 2h, use a dialysis bag (MWCO=3500da) to dialyze for 48h to prepare drug-loaded nanoparticles, such as figure 1 .
[0047] 2) Preparation of erythrocyte membrane vesicles
[0048] Whole blood was centrifuged at 4°C (2000rpm, 10min) to remove serum. Red blood cells were washed with phosphate buffered saline (PBS, 3 times) pH=7.4. Red blood cells were then resuspended in 0.25×PBS (pH=7.4) containing...
Embodiment 2
[0054] A preferred embodiment of the present invention provides a method for preparing a biomimetic modified valve material, specifically a method for preparing platelet membrane-wrapped nano drug-loaded particles for bionic modification of porcine pericardial valves, which is obtained by the following steps:
[0055] 1) Preparation of drug-loaded nanoparticles
[0056] PLGA (10 mg, MW.50000, 50:50), a hydrophobic anti-inflammatory drug (1 mg) was dissolved in 1 mL of dimethyl sulfoxide (DMSO). Then the mixed solution was added dropwise to 3 mL of water and stirred gently. After 2 hours, use a dialysis bag (MWCO=3500da) to dialyze for 48 hours to prepare drug-loaded nanoparticles.
[0057] 2) Preparation of platelet membrane
[0058] First, whole blood was centrifuged at 1600 rpm for 20 minutes to obtain platelets. Platelets were washed with phosphate buffered saline (PBS, 3 times) pH=7.4. Platelets were then resuspended in 0.25×PBS (pH=7.4) containing EDTAK2 at 4°C. Afte...
Embodiment 3
[0064] A preferred embodiment of the present invention provides a method for preparing a biomimetic modified valve material, specifically a method for preparing macrophage membrane-wrapped nano drug-loaded particles for bionic modification of porcine pericardial valves, which is obtained by the following steps:
[0065] 1) Preparation of drug-loaded nanoparticles
[0066] PLGA (10 mg, MW.50000, 50:50), hydrophobic anticoagulant drug (1 mg) was dissolved in 1 mL dimethyl sulfoxide (DMSO). Then the mixed solution was added dropwise to 3 mL of water and stirred gently. After 2 hours, use a dialysis bag (MWCO=3500da) to dialyze for 48 hours to prepare drug-loaded nanoparticles.
[0067] 2) Preparation of macrophage membrane
[0068] Macrophages are first cultured and collected. Then, macrophages were resuspended in 0.25×PBS (pH=7.4) at 4°C. After 30 min, subcellular organelles such as macrophage nuclei were removed by gradient density centrifugation, and macrophage membranes w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com