Preparation method of dexzopiclone photodegradation impurity
A technology of compound and feeding amount, applied in organic chemistry methods, organic chemistry and other directions, can solve problems such as danger and long route steps, and achieve the effects of low cost, easy operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
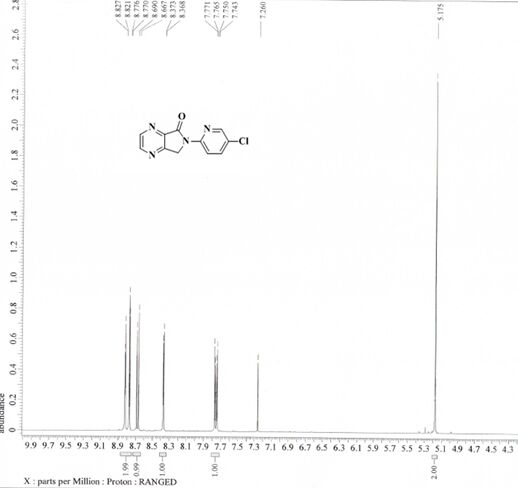
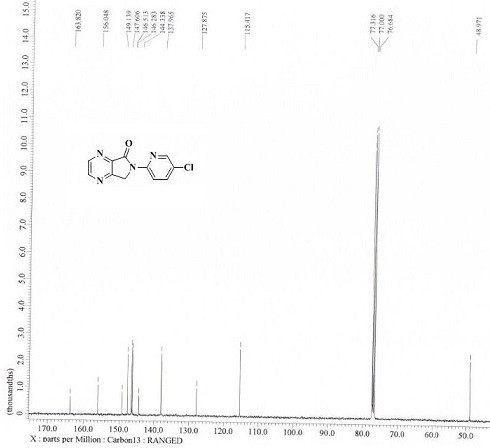
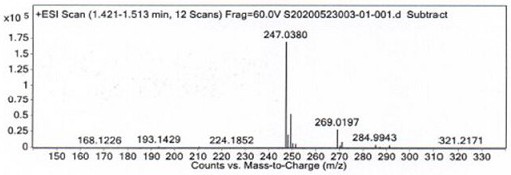
[0033] Example 1: 6-(5-chloropyridin-2-yl)-6,7-dihydro-5 H -pyrrolo[3,4- b ] Preparation of pyrazin-5-one (compound Ⅰ)
[0034] Add 6-(5-chloropyridin-2-yl)-7-hydroxyl-6,7-dihydro-5 H -pyrrolo[3,4- b ]pyrazin-5-one (compound Ⅱ, 10.00g, 0.0381mol ), 100.0ml toluene, stirred and cooled to -15°C, controlled temperature -15°C, slowly added diisobutylaluminum hydride (11.50ml, 0.0172mol ), after dropping, keep warm at -15°C for 2h. After the reaction is completed, add 50.0ml of methanol dropwise at a temperature below 0°C, stir for 1 hour after the dropwise addition, pour the reaction solution into 1000 ml of 1N hydrochloric acid at 3°C and stir for 1 hour, filter with suction, wash the filtrate with 100.0ml of drinking water, and organically Evaporate to dryness under reduced pressure at a vacuum degree of -0.10MPa in a water bath at 60°C, add 100.0ml of petroleum ether / ethyl acetate (ethyl acetate:petroleum ether=1:5) mixed solution, keep stirring at -10°C for 2h, Suction ...
Embodiment 2
[0036] Add (6-(5-pyridin-2-yl)-5 H -Pyrrole [3,4- b ]pyrazine-5,7(6 H )-diketone (Compound III, 10.00g, 0.0384mol) and 100.0ml of toluene were stirred and cooled to -10~-15°C, and the temperature was controlled at -10°C, and diisobutylaluminum hydride (23.10ml, 0.0346mol) was slowly added dropwise, After dropping, keep warm at -10°C for 2 hours. After the reaction is completed, add 50.0ml of methanol dropwise at a temperature below 0°C, stir for 1 hour after the dropwise addition, pour the reaction solution into 1000 ml of 1N hydrochloric acid at 5°C and stir for 0.5 hours, filter with suction, and wash the filtrate with 100.0ml of drinking water. The organic phase was evaporated to dryness in a water bath at 60°C with a vacuum degree of -0.10MPa, added 100.0ml of petroleum ether / ethyl acetate (ethyl acetate:petroleum ether=1:5) mixed solution, and kept at -10°C to stir and crystallize for 2h , suction filtered, and dried at 50°C to give 6-(5-chloropyridin-2-yl)-6,7-dihydro...
Embodiment 3
[0038] Add 6-(5-chloropyridin-2-yl)-7-hydroxyl-6,7-dihydro-5 H -pyrrolo[3,4- b]Pyrazin-5-one (Compound Ⅱ, 10.00g, 0.0381mol) and 100.0ml xylene were cooled to -12°C with stirring, and diisobutylaluminum hydride (7.70ml, 0.0114mol) was slowly added dropwise under temperature control at -12°C After dropping, keep warm at -12°C for 2h. After the reaction is completed, add 50.0ml of methanol dropwise at a temperature below 0°C, stir for 0.5h after the dropwise addition, pour the reaction solution into 1000ml of 1 N hydrochloric acid at 4°C and stir for 1h, filter with suction, and wash the filtrate with 100.0ml of drinking water , the organic phase was evaporated to dryness in a water bath at 50°C with a vacuum degree of -0.08MPa, added 100.0ml of petroleum ether / ethyl acetate (acetic acid:petroleum ether=1:5) mixed solution, and kept stirring at -5°C for 2h , suction filtered, and dried at 40°C to give 6-(5-chloropyridin-2-yl)-6,7-dihydro-5 H -pyrrolo[3,4- b ] Pyrazin-5-one 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com