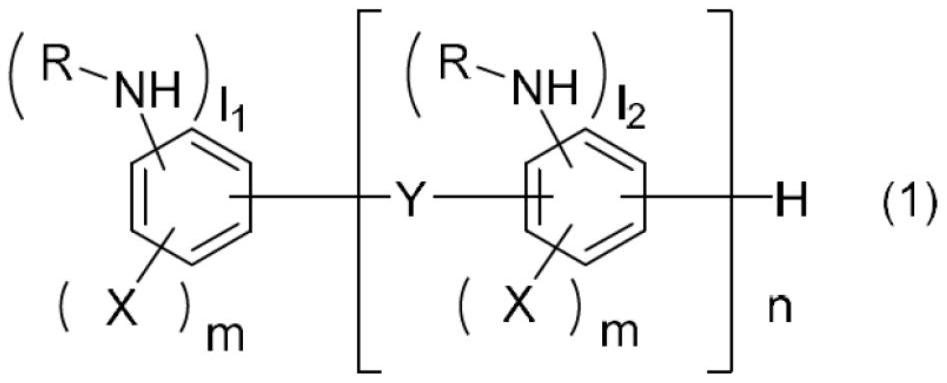
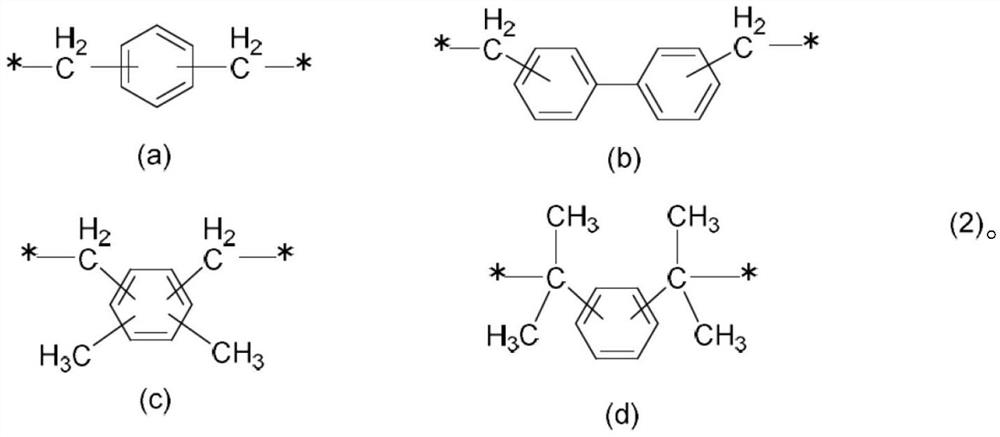
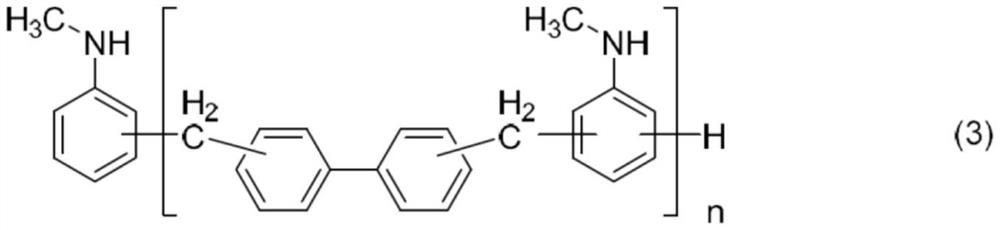
Aromatic amine resin having n-alkyl group, curable resin composition, and cured product thereof
A kind of hardening resin, aromatic amine technology, used in condensation/addition reaction to prepare amino compounds, organic chemistry, etc., can solve the problems of insufficient hygroscopicity or electrical properties, insufficient reactivity, unsatisfactory electrical properties, etc. , to achieve the effect of excellent hardenability and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] 150 g of toluene and 56.0 g of N-methylaniline were added to a flask equipped with a thermometer, a cooling pipe, and a stirrer, and stirred. Then, 43.5 g of 4,4'- bischloromethylene biphenyl was added over 1 hour, and it was made to react at 65 degreeC for 2 hours. Next, 36.1 g of 35% hydrochloric acid was added dropwise at 80°C or lower. After completion of the dropwise addition, the temperature was raised to 210° C. while azeotropic dehydration, and the reaction was carried out at 210° C. for 15 hours. After the reaction, 100 g of 17% caustic soda aqueous solution was added dropwise to make the reaction solution alkaline, and 100 g of toluene and 150 g of N-methylaniline were added thereto, followed by stirring for 6 hours. After standing still and waste water, wash with water until the waste water becomes neutral. Solvent, water, unreacted N-methylaniline were distilled off by concentration under reduced pressure, and biphenyl-N-methylaniline novolac (BNMAN-1) was...
Embodiment 2
[0170] 50 g of toluene and 171.5 g of N-methylaniline were added to a flask equipped with a thermometer, a cooling pipe, and a stirrer, and stirred. Then, 50.2 g of 4,4'- bischloromethylene biphenyl was added over 1 hour, and it was made to react at 65 degreeC for 2 hours. Next, 41.7 g of 35% hydrochloric acid was added dropwise at 80° C. or lower. After completion of the dropwise addition, the temperature was raised to 210° C. while azeotropic dehydration, and the reaction was carried out at 210° C. for 15 hours. After completion of the reaction, 40 g of 48% caustic soda aqueous solution was added dropwise to make the reaction liquid alkaline, and 50 g of toluene was further added, followed by stirring for 6 hours. After standing still and waste water, wash with water until the waste water becomes neutral. Solvent, water, unreacted N-methylaniline were distilled off by concentrating under reduced pressure, and biphenyl-N-methylaniline novolac (BNMAN-2) was obtained in the f...
Embodiment 3
[0172] 200 g of water and 214 g of N-methylaniline were added to a flask equipped with a thermometer, a cooling pipe, and a stirrer, and stirred. Then, 209 g of 35% hydrochloric acid was dripped at 40 degreeC or less. Next, 108 g of 37% formaldehyde aqueous solution was dripped at 40 degreeC or less. After completion of the dropwise addition, the mixture was reacted at 75°C to 80°C for 5 hours. After completion of the reaction, 168 g of 48% caustic soda aqueous solution was added dropwise to make the reaction liquid alkaline, and 300 g of toluene was further added, followed by stirring for 6 hours. After standing still and waste water, wash with water until the waste water becomes neutral. The solvent, water, and unreacted N-methylaniline were distilled off by concentration under reduced pressure to obtain 200 g of N-methylaniline novolac (NMAN-1) in the form of a black liquid resin (amine equivalent: 116 g / eq., composition The ratio is GPC area %: n=1 component: 37%, n=2 c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Amine equivalent | aaaaa | aaaaa |
| Softening point | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com