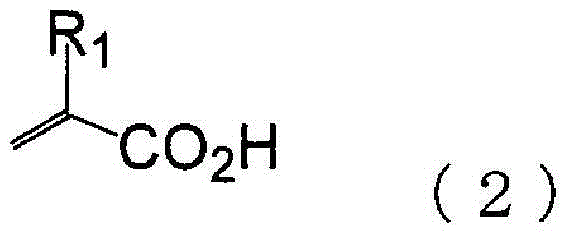Thermosetting resin composition containing (meth)acrylate resin and cured product using the thermosetting resin composition
A technology of thermosetting resin and acrylate, applied in the direction of coating, optical filter, etc., can solve the problem of insufficient adhesion of the protective film, and achieve the effect of excellent adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
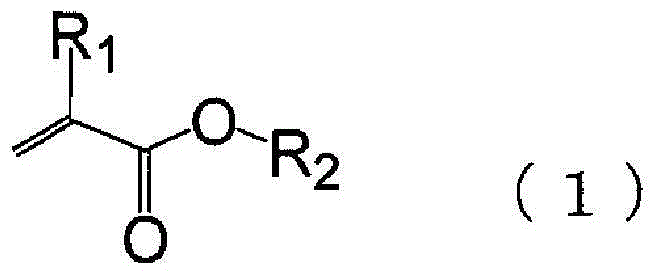
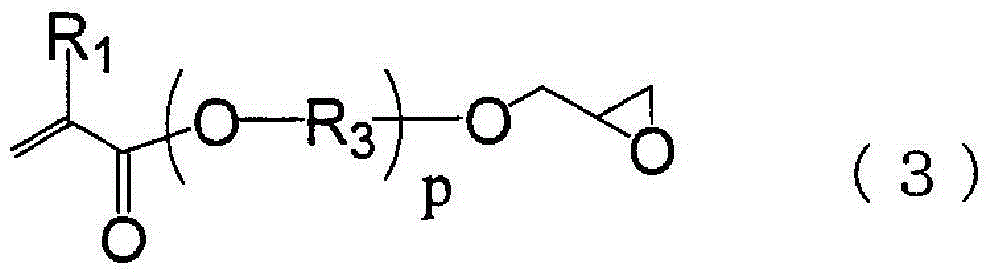
[0096] In a 1000ml four-necked flask with a nitrogen inlet tube and a reflux tube, 51.65g (0.60mol) of methacrylic acid, 38.44g (0.38mol) of methyl methacrylate, and 36.33g (0.22mol) of cyclohexyl methacrylate were charged. mol), 5.91g (0.036mol) of AIBN, and 368g of DMDG were stirred at 80-85°C for 8 hours under nitrogen flow to polymerize. In addition, 39.23 g (0.28 mol) of glycidyl methacrylate, 1.44 g (0.0055 mol) of TPP, and 0.055 g of 2,6-di-tert-butyl-p-cresol were placed in the flask, and stirred at 80-85° C. for 16 hours. , and (meth)acrylate resin (i)-1 containing a polymerizable double bond was obtained. The obtained polymerizable double bond-containing (meth)acrylate resin had a solid content concentration of 32.0% by mass, an acid value (in terms of solid content) of 110 mgKOH / g, and a Mw of 18080 by GPC analysis. In addition, from the IR measurement of the obtained polymerizable double bond-containing (meth)acrylate resin, it was observed that -1 (vinyl), 1186c...
Synthetic example 2
[0098] In a 1000ml four-necked flask with a nitrogen inlet tube and a reflux tube, 51.65g (0.60mol) of methacrylic acid, 38.44g (0.38mol) of methyl methacrylate, and 36.33g (0.22mol) of cyclohexyl methacrylate were charged. mol), 7.39g (0.045mol) of AIBN, and 371g of DMDG were stirred at 80-85°C for 8 hours under nitrogen flow to polymerize. In addition, 39.23 g (0.28 mol) of glycidyl methacrylate, 1.44 g (0.0055 mol) of TPP, and 0.055 g of 2,6-di-tert-butyl-p-cresol were placed in the flask, and stirred at 80-85° C. for 16 hours. , and (meth)acrylate resin (i)-2 containing a polymerizable double bond was obtained. The obtained polymerizable double bond-containing (meth)acrylate resin had a solid content concentration of 31.5% by mass, an acid value (in terms of solid content) of 109 mgKOH / g, and a Mw of 14400 by GPC analysis. In addition, from the IR measurement of the obtained polymerizable double bond-containing (meth)acrylate resin, it was observed that at 1410 cm -1 (vi...
Synthetic example 3
[0100] In a 1000ml four-necked flask with a nitrogen inlet pipe and a reflux pipe, 51.65g (0.60mol) of methacrylic acid, 38.44g (0.38mol) of methyl methacrylate, and 36.34g (0.22 mol), 3.35g (0.020mol) of AIBN, and 363g of DMDG were stirred at 80-85°C for 8 hours under nitrogen flow to polymerize. In addition, 37.53 g (0.26 mol) of glycidyl methacrylate, 1.38 g (0.0053 mol) of TPP, and 0.053 g of 2,6-di-tert-butyl-p-cresol were placed in the flask, and stirred at 80-85° C. for 16 hours. , to obtain a polymerizable double bond-containing (meth)acrylate resin (i)-3. The obtained polymerizable double bond-containing (meth)acrylate resin had a solid content concentration of 31.7% by mass, an acid value (in terms of solid content) of 117 mgKOH / g, and a Mw of 28280 by GPC analysis. In addition, from the IR measurement of the obtained polymerizable double bond-containing (meth)acrylate resin, it was observed that -1 (vinyl), 1185cm -1 (Carboxyl) has a peak. From this, it was conf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com