One-step method for preparing surface-functionalized superpara-Fe3O4 magnetic nanoparticles
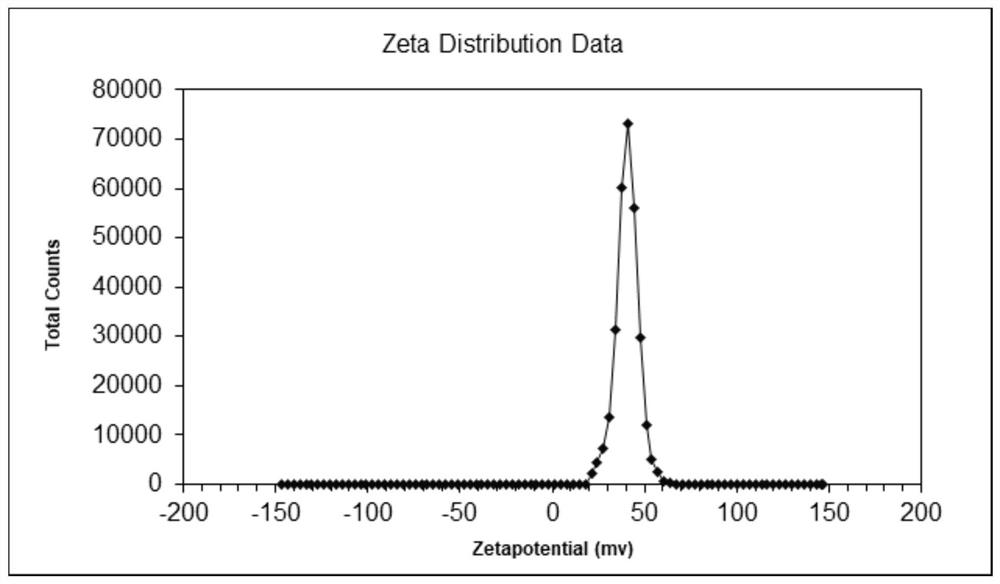
A technology of magnetic nanoparticles and surface functionalization, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve the problems of difficult removal of surfactants, cost, many steps, and complicated processes, etc., to achieve Increase stability, good dispersion, and avoid agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
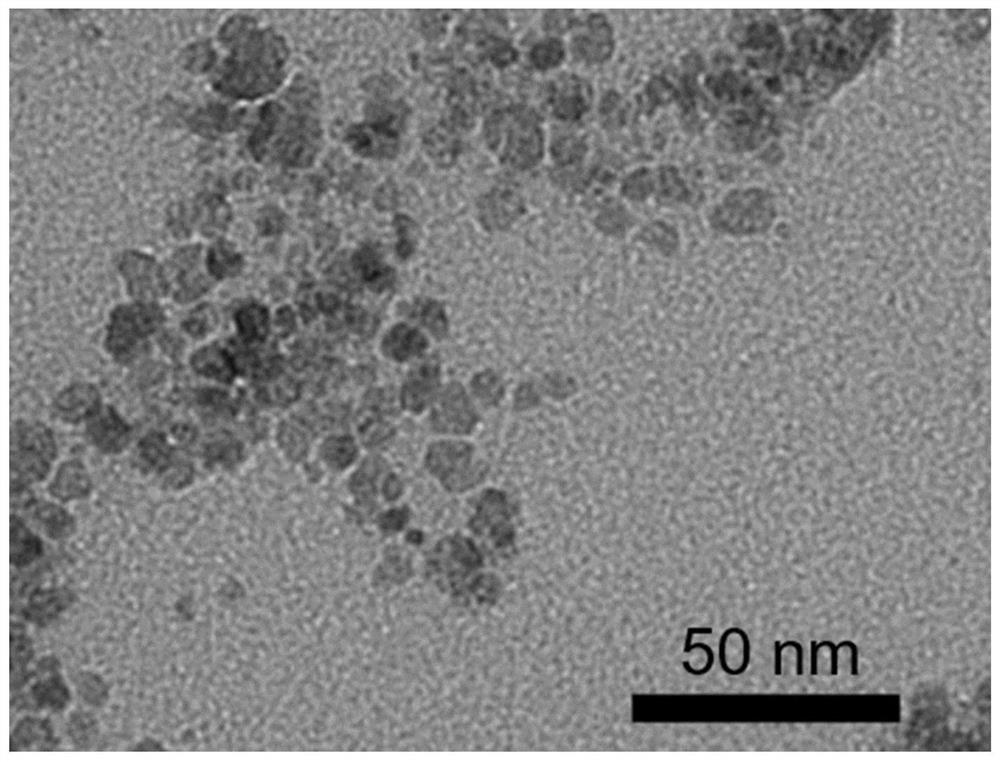
[0022] Embodiment 1 A kind of particle diameter 5nm, the Fe of tool propyl ammonium functionalized surface protection 3 o 4 Preparation of Magnetic Nanoparticles.
[0023] 1) Dissolve 0.2mol ferric chloride and 0.15mol ferrous chloride in 200ml DI H 2 O, placed in a 500ml three-neck flask and purged with nitrogen to remove the air for 20 minutes.
[0024] 2) Dissolve 0.9mol tetrapropylammonium hydroxide in 200ml DI H 2 O, nitrogen gas was passed for 20 minutes, and then added dropwise to a three-necked flask while nitrogen gas was passed and mechanical stirring was carried out. The reaction temperature was 20°C.
[0025] 3) Continue to stir for 4 hours after the dropwise addition, and wash the product three times with deionized water de-aired with nitrogen. Finally, the prepared magnetic particles are collected with a permanent magnet and stored in an aqueous solution to obtain Fe particles with a particle size of 5 nm and surface protection with propylammonium functionali...
Embodiment example 2
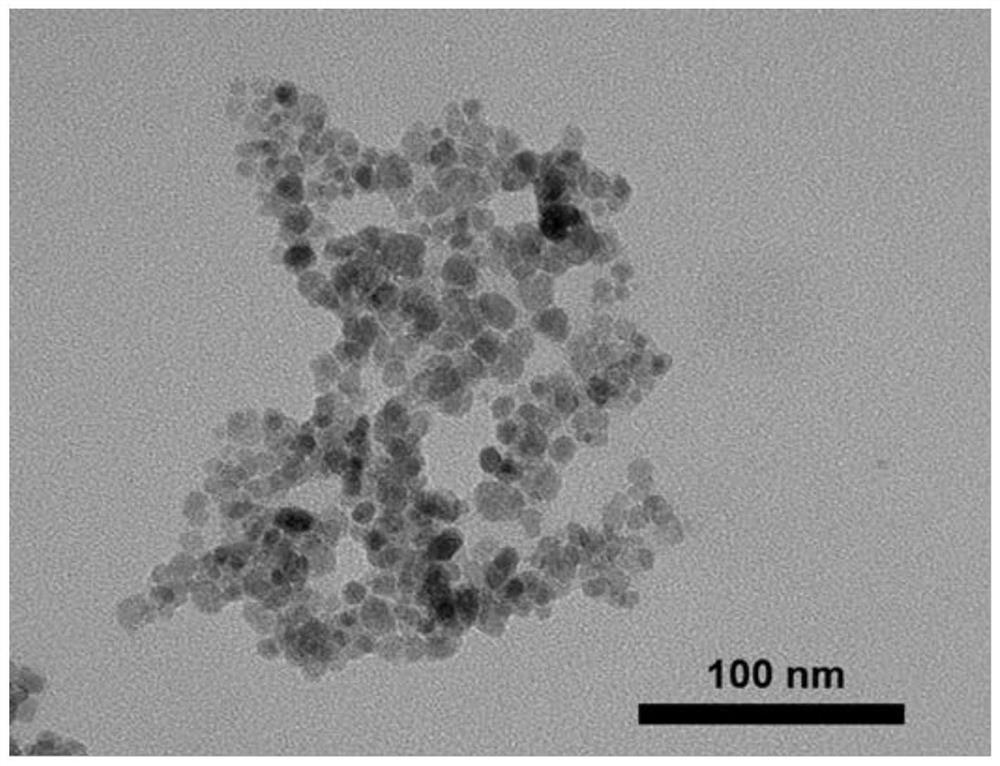
[0026] Example 2: Fe with a particle size of 10nm and surface protection with butyl ammonium 3 o 4 Preparation of Magnetic Nanoparticles.
[0027] 1) Dissolve 0.35mol ferric sulfate and 0.25mol ferrous sulfate in 150ml DI H 2 O, placed in a 500ml three-neck flask and passed through nitrogen to remove the air for 25 minutes.
[0028] 2) Dissolve 1.5mol tetrabutylammonium hydroxide in 150ml DI H 2 O, nitrogen gas was passed for 25 minutes, and then added dropwise to a three-necked flask while nitrogen gas was passed and mechanical stirring was carried out. The reaction temperature was 25°C.
[0029] 3) Continue to stir for 5.5 hours after the dropwise addition, and wash the product three times with deionized water de-aired with nitrogen. Finally, the prepared magnetic particles are collected with a permanent magnet and stored in an aqueous solution to obtain Fe with a particle size of 10 nm and surface protection with butylammonium. 3 o 4 magnetic nanoparticles.
Embodiment example 3
[0030] Example 3: Fe with a particle size of 10nm and surface protection with methylaniline functionalization 3 o 4 Preparation of Magnetic Nanoparticles.
[0031] 1) Dissolve 0.7mol ferric nitrate and 0.45mol ferrous nitrate in 200ml DI H 2 O, placed in a 500ml three-neck flask and purged with nitrogen to remove the air for 30 minutes.
[0032] 2) Dissolve 3.5mol trimethylphenylammonium hydroxide in 200ml DI H 2 O, nitrogen gas was passed for 30 minutes, and then added dropwise to a three-necked flask while nitrogen gas was passed and mechanical stirring was carried out. The reaction temperature was room temperature 22°C.
[0033] 3) Continue to stir for 6 hours after the dropwise addition, and wash the product three times with deionized water de-aired with nitrogen. Finally, the prepared magnetic particles are collected with a permanent magnet and stored in an aqueous solution to obtain Fe with a particle size of 10 nm and a surface protection with methylaniline function...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com