Fluorine-boron skeleton-based fluorescent probe TQBF-NBD with large Stokes displacement, and preparation method and application of fluorescent probe TQBF-NBD
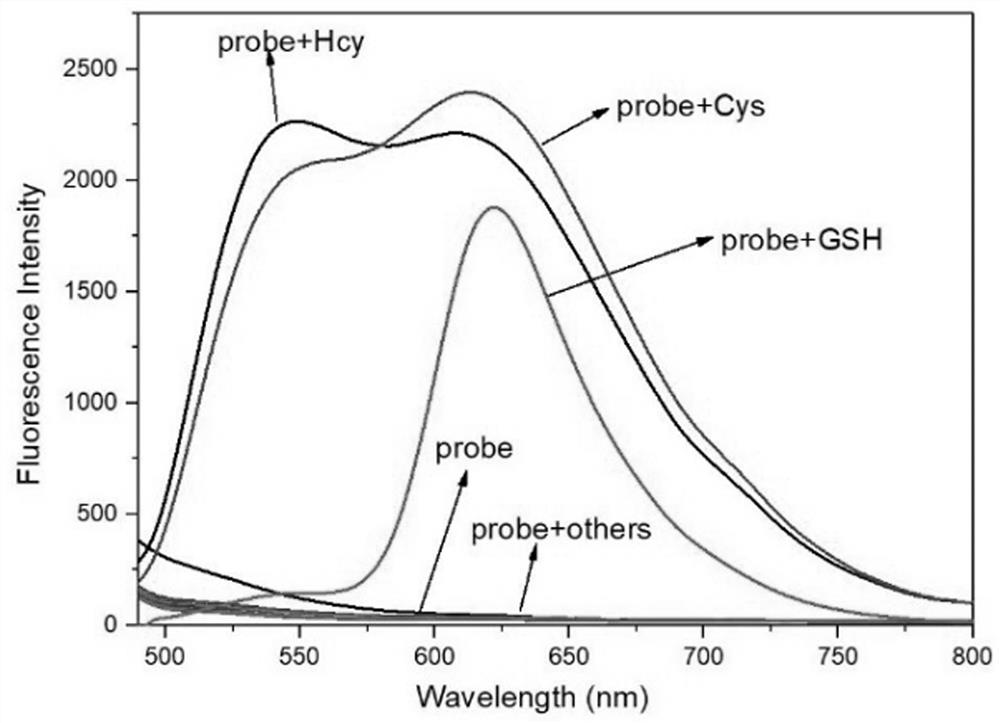
A technology of TQBF-NBD and TQBF-OH, applied in the field of fluorescent probes, can solve the problems of low detection sensitivity and separate detection of biothiols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
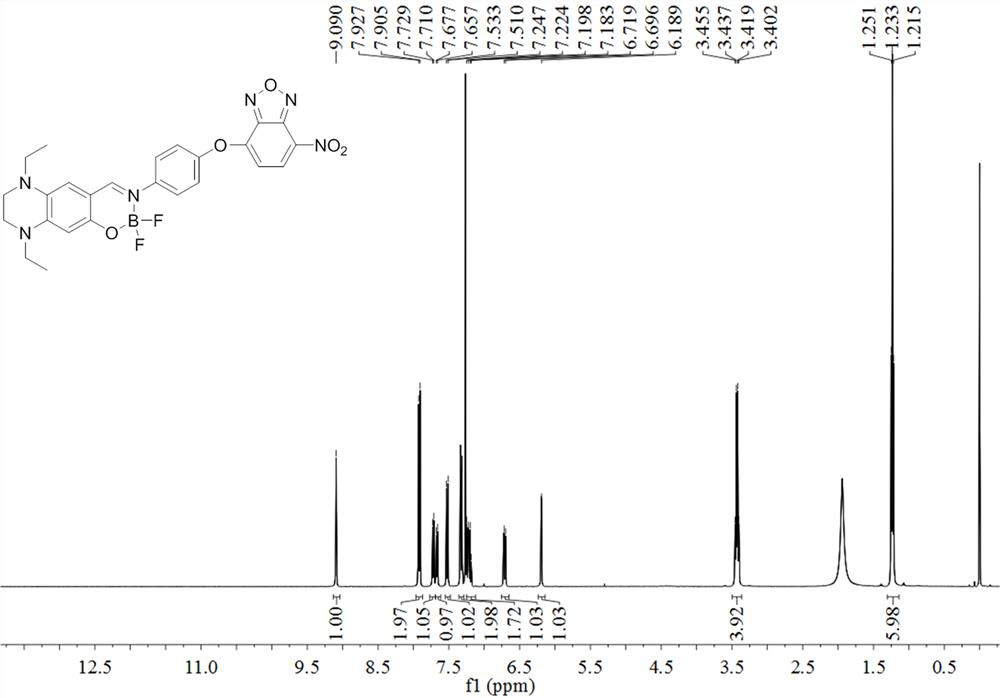
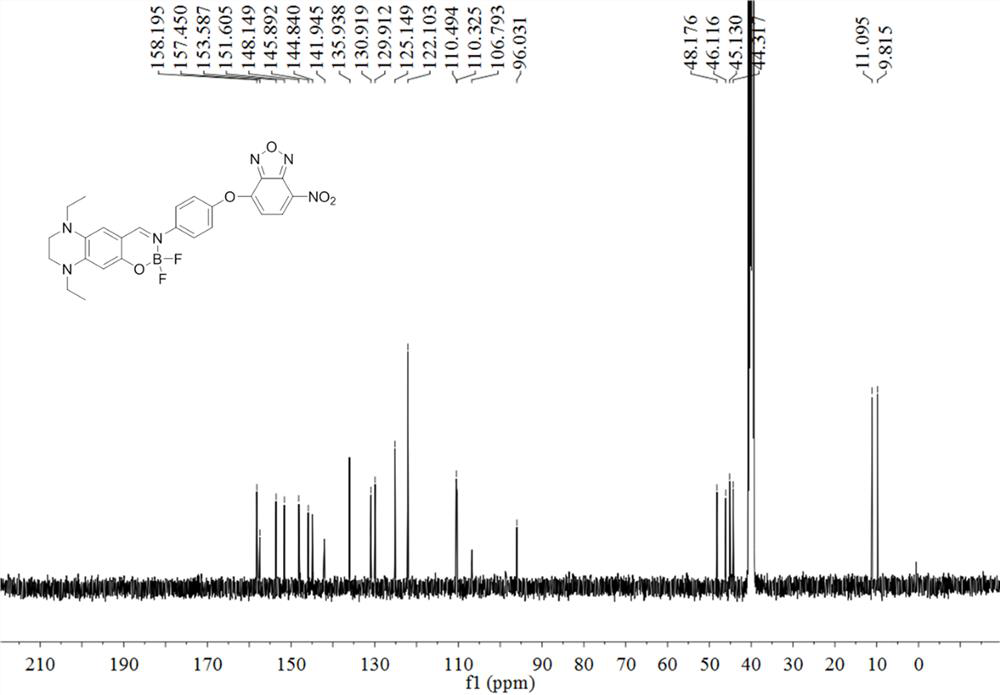
[0037] The preparation method of the fluorescent probe TQBF-NBD based on the large Stokes shift of the fluorine boron skeleton, the technical route is as follows:
[0038]
[0039] The preparation steps are as follows:
[0040] (a) Dissolve 2,4-diethyl-1,2,3,4-tetrahydroquinoxaline salicylaldehyde (234 mg, 1.0 mmol), p-aminophenol (121 mg, 1.2 mmol) and a catalytic amount of TsOH In 5 mL of ethanol, heated to reflux under the protection of argon for 2 hours, stopped the reaction, cooled the reaction solution to room temperature, and spin-dried to obtain a dark brown oily liquid crude product;
[0041] (b) Dissolve 200 μL of crude product in 10 mL 1,2-dichloroethane, then add 100 μL DIEA and 100 μL BF 3 •OEt 2 , reacted at 80 °C for 4 h under the protection of argon, stopped the reaction, added 30 mL of 1,2-dichloroethane to the reaction liquid, poured it into 100 mL of NaHCO 3 In the solution, the organic phase was separated, the aqueous phase was extracted 3 times with ...
Embodiment 2
[0045] The preparation method of the fluorescent probe TQBF-NBD based on the large Stokes shift of the fluorine boron skeleton, the technical route is as follows:
[0046]
[0047] The preparation steps are as follows:
[0048] (a) Dissolve 2,4-diethyl-1,2,3,4-tetrahydroquinoxaline salicylaldehyde (234 mg, 1.0 mmol), 1 mmol of p-aminophenol and 0.05 mmol of TsOH in 5 mL of ethanol , heated to reflux for 2 hours under argon protection, stopped the reaction, cooled the reaction solution to room temperature, and spin-dried to obtain a dark brown oily liquid crude product;
[0049] (b) Dissolve 250 μL of crude product in 10 mL 1,2-dichloroethane, then add 100 μL DIEA and 100 μL BF 3 •OEt 2 , reacted at 80 °C for 4 h under the protection of argon, stopped the reaction, added 30 mL of 1,2-dichloroethane to the reaction liquid, poured it into 100 mL of NaHCO 3 In the solution, the organic phase was separated, the aqueous phase was extracted 3 times with dichloromethane (3 × 30 ...
Embodiment 3
[0052] The preparation method of the fluorescent probe TQBF-NBD based on the large Stokes shift of the fluorine boron skeleton, the technical route is as follows:
[0053]
[0054] The preparation steps are as follows:
[0055] The preparation steps are as follows:
[0056] (a) Dissolve 2,4-diethyl-1,2,3,4-tetrahydroquinoxaline salicylaldehyde (234 mg, 1.0 mmol), 1.1 mmol of p-aminophenol and 0.05 mmol of TsOH in 5 mL In ethanol, heated to reflux for 2 hours under the protection of argon, the reaction was stopped, the reaction solution was cooled to room temperature, and spin-dried to obtain a dark brown oily liquid crude product;
[0057] (b) Dissolve 230 μL of the crude product in 10 mL of 1,2-dichloroethane, then add 100 μL of DIEA and 100 μL of BF 3 •OEt 2 , reacted at 80 °C for 4 h under the protection of argon, stopped the reaction, added 30 mL of 1,2-dichloroethane to the reaction liquid, poured it into 100 mL of NaHCO 3 In the solution, the organic phase was sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com