Preparation method for protein fixed-point pegylation modification
A technology of PEGylation and polyethylene glycol, applied in the field of protein modification, to achieve high modification yield, simple termination process, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] rhG-CSF reaction solution (40ml, concentration 3mg / ml), containing 100mM sodium phosphate, pH5.0, also contains 120mM MVB6 (vitamin B6), after it is fully stirred and frozen (4 ℃), add 5 times the amount of protein Methoxypolyethylene glycol propionaldehyde (MPEG-PALD) (average molecular weight 20 kDa). The reaction mixture was continuously stirred at the same temperature.
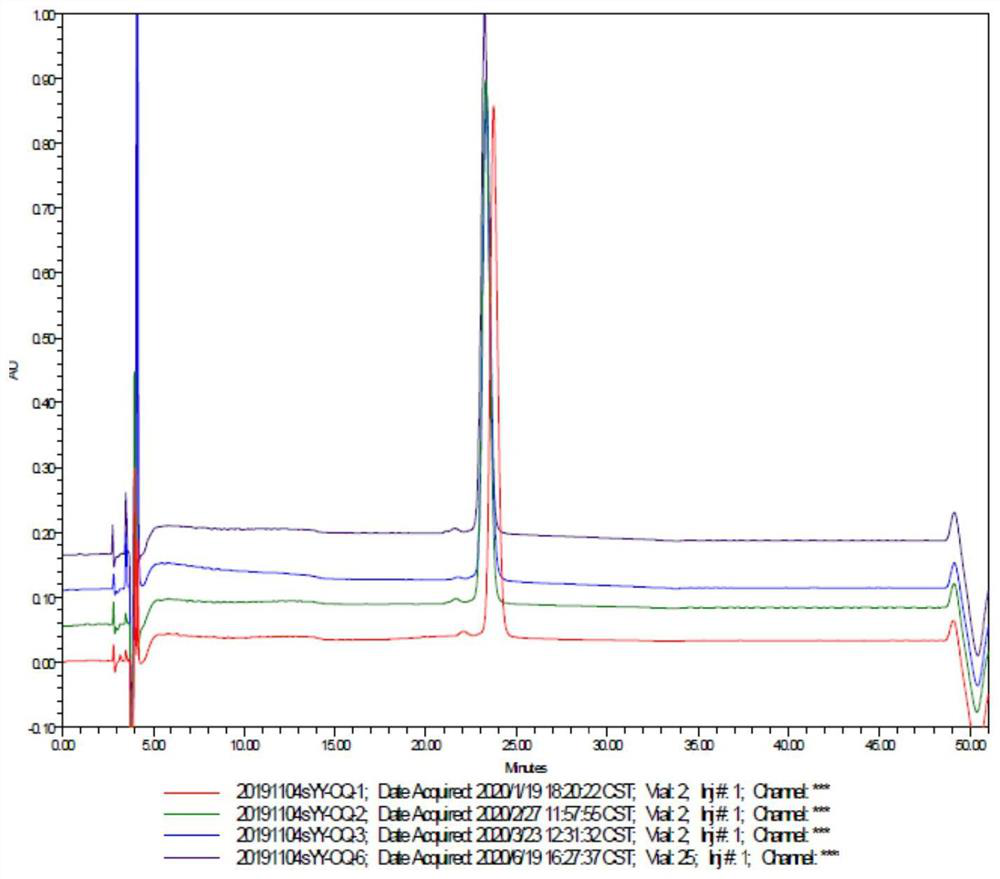
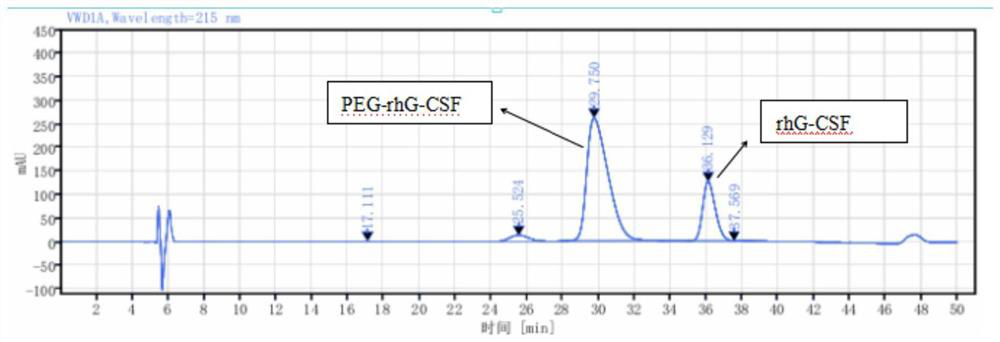
[0048] During the reaction, the modification rate of protein was monitored by RP-HPLC. What RP-HPLC used was a C18 250×4.6mm 5 μm column (phenomenex), with 0.1% trifluoroacetic acid aqueous solution and 0.1% trifluoroacetic acid acetonitrile at 1.0 Gradient elution was performed at a flow rate of ml / min.
[0049] The RP-HPLC analysis results at 8, 9, 10, and 11 hours respectively are shown in Table 1:
[0050]
[0051]
Embodiment 2
[0053] rhG-CSF reaction solution (40ml, concentration 3mg / ml), containing 100mM sodium phosphate, pH5.0, also contains 40mM NADPH (nicotinamide adenine dinucleotide phosphate), fully stirred and frozen (4 ℃) , Add 5 times the protein amount of methoxypolyethylene glycol propionaldehyde (MPEG-PALD) (average molecular weight is 20kDa). The reaction mixture was continuously stirred at the same temperature.
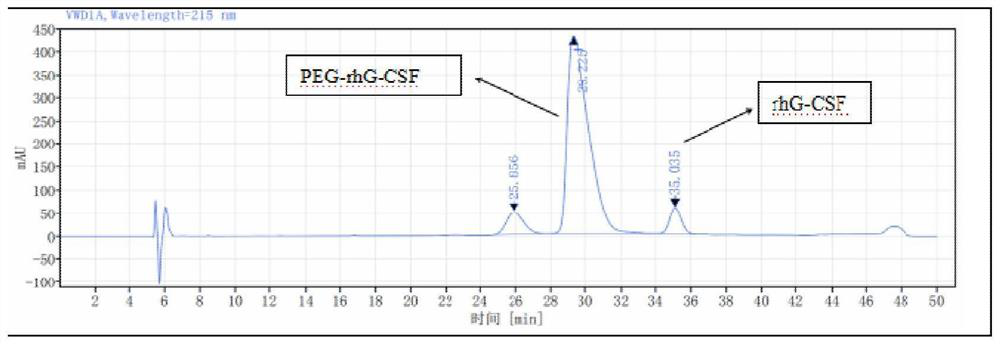
[0054] During the reaction, the modification rate of protein was monitored by RP-HPLC. What RP-HPLC used was a C18 250×4.6mm 5 μm column (phenomenex), with 0.1% trifluoroacetic acid aqueous solution and 0.1% trifluoroacetic acid acetonitrile at 1.0 Gradient elution was performed at a flow rate of ml / min.
[0055] The RP-HPLC analysis results at 8, 9, 10, and 11 hours respectively are shown in Table 1:
[0056] time RP-HPLC modification rate (%) 8 77.5 9 84.4 10 86.0 11 85.8
Embodiment 3
[0058] rhG-CSF reaction solution (40ml, concentration 3mg / ml), containing 300mM sodium phosphate, pH 7.0, also contains 40mM NADPH (nicotinamide adenine dinucleotide phosphate), fully stirred and frozen (4 ℃) , Add 5 times the protein amount of methoxypolyethylene glycol propionaldehyde (MPEG-PALD) (average molecular weight is 20kDa). The reaction mixture was continuously stirred at the same temperature.
[0059] During the reaction, the modification rate of protein was monitored by RP-HPLC. What RP-HPLC used was a C18 250×4.6mm 5 μm column (phenomenex), with 0.1% trifluoroacetic acid aqueous solution and 0.1% trifluoroacetic acid acetonitrile at 1.0 Gradient elution was performed at a flow rate of ml / min.
[0060] The RP-HPLC analysis results at 8, 9, 10, and 11 hours respectively are shown in Table 1:
[0061] time RP-HPLC modification rate (%) 8 72.3 9 75.1 10 79.5 11 80.8
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com