ShRNA for inhibiting replication of SARS-COV-2 virus and application of shRNA
A virus replication, sars-cov-2 technology, applied in DNA/RNA fragments, antiviral agents, recombinant DNA technology, etc., can solve problems such as economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 Interference effect of shRNA carried by adeno-associated virus
[0147] virus strain
[0148] The novel coronavirus (SARS-CoV-2) strain (GenBank: MT123290) was isolated from a throat swab of a patient and kept in the P3 laboratory of Guangzhou Customs Technical Center.
[0149] Reagent material
[0150] Vero E6 cells, 96-well cell culture plate, DMEM medium, 2% bovine serum DMEM medium, primary antibody, secondary antibody, etc. Freshly prepared 10% hypochlorous acid solution, 4% paraformaldehyde, 1.6% CMC.
[0151] experimental method
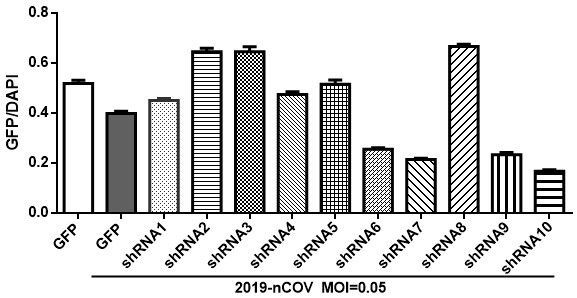
[0152] Take Vero E6 cells susceptible to SARS-COV-2, according to 1*10 4 per well into a 96-well plate. After 24 hours of wall attachment, each well was transfected with lipofectamine3000 reagent according to the amount of 0.25 μg shRNA contained in adeno-associated virus. After 24 h, the transfection efficiency was observed.
[0153] Bring the 96-well plate into P3, and add SARS-COV-2 at a dose (non-lethal dose) with a...
Embodiment 2
[0170] Example 2 The interference effect of lentivirus-encapsulated shRNA
[0171] experimental method
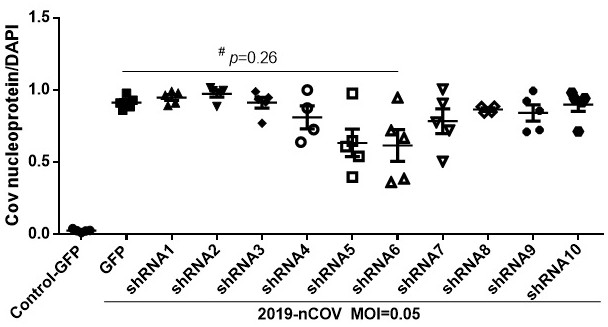
[0172] Infect the Vero E6 cells with the packaged lentivirus respectively, and select the stably transfected cells by puromycin resistance, according to 1.5*10 4 One per well was placed in a 96-well plate, and after 24 hours of attachment, SARS-COV-2 was added at a dose (non-lethal dose) of 0.05 at the multiplicity of infection (MOI). The infection efficiency was determined by immunofluorescence (as described in Example 1).
[0173] Experimental results
[0174] Such as image 3 and 4 As shown, compared with the control, the interference effects of shRNA5, shRNA6, shRNA7, shRNA9, and shRNA10 carried by the lentivirus are extremely significant.
Embodiment 3
[0175] Example 3 Effect of shRNA on viral replication
[0176] experimental method
[0177] Vero E6 cell lines stably transfected with different shRNAs were used at 1×10 per well 5 Cells were plated in 24-well plates, with 4 replicates of each shRNA, and GFP was used as a control. Inoculate SARS-CoV-2 according to MOI=0.05, 24 hours after infection, scrape off the cell culture supernatant and cells together, freeze and thaw once, and use Focus-forming Assay (FFA) to measure the virus in the cell culture Titer. Specific steps are as follows:
[0178] 1. Seed VeroE6 cells in 96-well flat-bottom plate, 2×104 cells / well.
[0179] 2. Wait until the cells reach 100% confluence
[0180] (1) Take 20 μl of the virus solution to be tested, serially dilute it 10 times with DMEM containing 2% FBS, and mix well.
[0181](2) Discard the cell culture medium, add virus supernatants of different dilutions to the cell wells, 50 µL / well, shake slightly to make the liquid evenly cover the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com