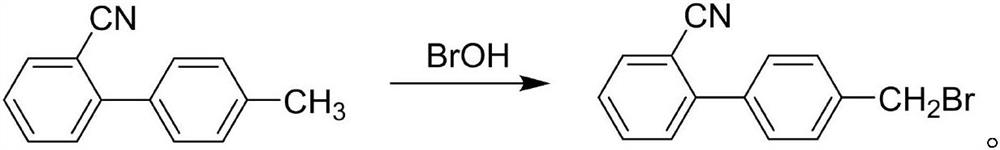
Preparation formula of 4'-bromomethyl-2-cyanobiphenyl
The technology of cyanobiphenyl and bromomethyl is applied in the field of preparation formula of 4'-bromomethyl-2-cyanobiphenyl, and can solve the problems of reducing the bromination reaction speed, slow reaction speed, equipment corrosion and the like, To achieve the effect of easy industrial production, mild reaction conditions, and low equipment corrosiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation formula of 4'-bromomethyl-2-cyanobiphenyl according to the present invention is made of the following raw materials in parts by weight: 550-650 parts of organic solvent, 550-650 parts of water, 4'-methanol 190-195 parts of base-2-cyanobiphenyl, 60-85 parts of bromide salt, 45-55 parts of bromate or chlorate, 150-250 parts of hydrochloric acid solution, and 450-550 parts of recrystallization solvent.
[0032] Further, the concentration of the hydrochloric acid is 15-30%.
[0033] Further, the recrystallization solvent is toluene or ethyl acetate or tert-butyl methyl ether or water.
[0034] Further, the recrystallization solvent is toluene.
[0035] Further, the bromide salt is sodium bromide or potassium bromide or lithium bromide or magnesium bromide or calcium bromide.
[0036] Further, the bromide salt is sodium bromide or potassium bromide.
[0037] Further, the bromate is sodium bromate or potassium bromate or lithium bromate or magnesium bromate o...
Embodiment 2
[0042] A preparation formula of 4'-bromomethyl-2-cyanobiphenyl according to the present invention is made of the following raw materials in parts by weight: 600 parts of organic solvent, 600 parts of water, 4'-methyl-2- 103 parts of cyanobiphenyl, 69 parts of bromide salt, 50 parts of bromate or chlorate, 200 parts of hydrochloric acid solution, and 500 parts of recrystallization solvent.
[0043] Further, the concentration of the hydrochloric acid is 15-30%.
[0044] Further, the recrystallization solvent is toluene or ethyl acetate or tert-butyl methyl ether or water.
[0045] Further, the recrystallization solvent is toluene.
[0046] Further, the bromide salt is sodium bromide or potassium bromide or lithium bromide or magnesium bromide or calcium bromide.
[0047] Further, the bromide salt is sodium bromide or potassium bromide.
[0048] Further, the bromate is sodium bromate or potassium bromate or lithium bromate or magnesium bromate or calcium bromate or sodium chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com