Kinase inhibitor compounds and compositions and methods of use
A technology for compounds, solvates, applied in the field of kinase inhibitor compounds and compositions and uses, which can solve problems such as the potential for drug development that limit therapeutic utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0272] Example 1 - Materials and methods used in Examples 2-5
[0273] 1 H and 13 C NMR spectrum. 1 H and 13 C NMR spectra were performed on a Bruker DRX-600 spectrometer for 1 H at 600MHz and for 13 C obtained at 150MHz. TLC was performed on aluminum plates coated with silica (200 μm in thickness) or aluminum oxide (200 μm in thickness) provided by Sorbent Technologies, and column chromatography was performed on a column equipped with a variable wavelength detector and fractionated Collector's Teledyne ISCO combiflash, using RediSep Rf high-efficiency silica flash column provided by Teledyne ISCO. LCMS analysis was performed on an Agilent Technologies G1969A high resolution API-TOF mass spectrometer connected to an Agilent Technologies 1200HPLC system. Samples were ionized in positive mode by electrospray ionization (ESI). Chromatography was performed on a 2.1×150 mm Zorbax300SB-C18 5-μm column with water containing 0.1% formic acid as solvent A and acetonitrile conta...
Embodiment 2
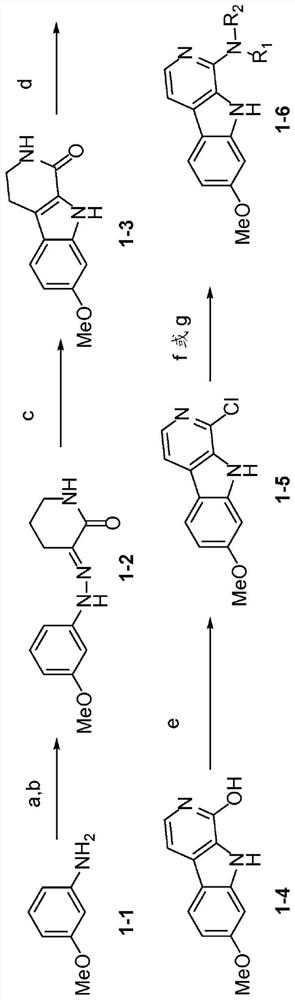
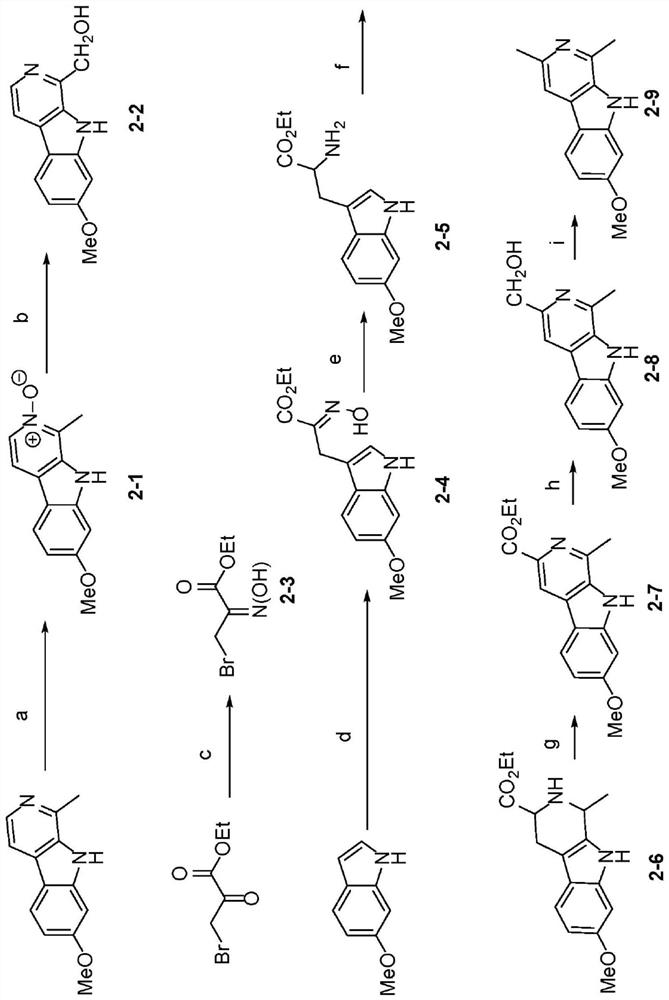
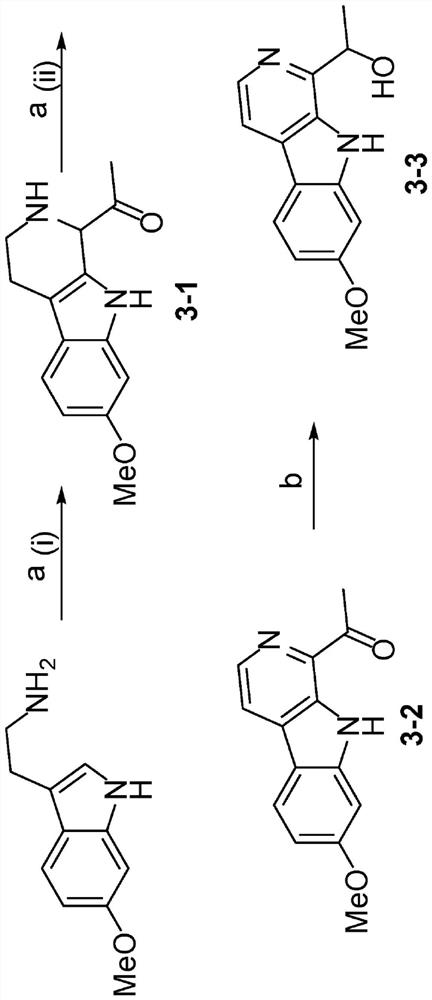
[0301] Example 2 - Chemical Synthesis of Selected Haraline Analogs
[0302] The 1-position harmine analog according to figure 1 Outlined reaction sequence for synthesis. The classical diazotization of m-anisidine to form the corresponding aryldiazonium salt, coupling of the aryldiazonium salt with 3-carboxy-2-piperidinone yields arylhydrazone 1-2 (Luis et al., "The Fischer Indole Synthesis of 8-Methyl-5-Substituted-1-Oxo-β-Carbolines: ARemarkable High Yield of a[1,2]-Methyl Migration," Tetrahedron 47(9):1737-44(1991), hereby incorporated by reference in its entirety). The resulting arylhydrazone was subjected to Fisher indole cyclization in the presence of formic acid to give 1,2,3,4-tetrahydro-1-oxo-β-carboline 1-3 (Luis et al., "The Fischer Indole Synthesis of 8-Methyl-5-Substituted-1-Oxo-β-Carbolines: A Remarkable High Yield of a[1,2]-Methyl Migration," Tetrahedron 4(9):1737-44( 1991), which is hereby incorporated by reference in its entirety). Oxidation of 1-3 using D...
Embodiment 3
[0305] Example 3 - Structure-Activity Relationship Study (SAR) of Selected Harmine Analogs
[0306] Introduction of structural modifications to the 1-position of harmine to identify novel harmine-based DYRK1A inhibitors that can be linked to GLP-1 agonists for β-cell-targeted delivery. according to Figure 1-2 According to the route described in , a total of 15 kinds of harmine analogs were synthesized. These analogs were initially screened for DYRK1A binding activity at 1000 nM and 300 nM using the FRET-based LanthaScreen binding assay (Life Technologies). Those compounds showing ≥50% inhibition at 300 nM were titrated using ten serial three-fold dilutions (in duplicate) to determine the IC 50 .
[0307] The effect of a cycloalkylamine at the 1-position of hamateline on DYRK1A binding was investigated. First, three analogs having azetidine (1-6a), pyrrolidine (1-6b) and piperidine (1-6c) substituents at the 1-position were synthesized. Compound 1-6a showed the best activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com