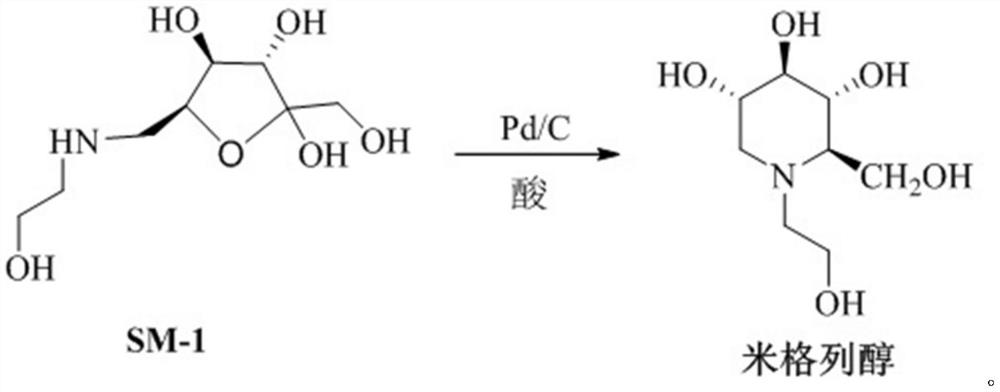
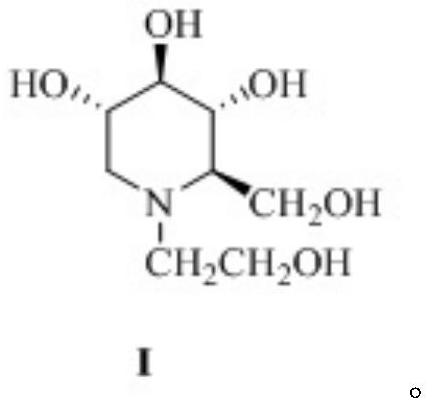
Preparation method of miglitol
A miglitol and reaction technology, applied in the field of miglitol preparation, can solve the problems of complicated operation, low product yield and purity, and achieve the effects of simple operation steps, high product purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In the autoclave, SM-1 (10.00g, 44.80mmol), Pd / C (0.5g), and acetic acid (5.38g, 89.6mmol) were added to the tetrahydrofuran / purified water system (V: 四氢呋喃 :V: 纯化水 =1:2, 500mL), stirring, temperature control 35 ~ 40 ° C, pressure control at 6 ± 0.5Mpa reaction 6h, the reaction is completed, the reaction solution is cooled to room temperature, and the solid concentrated under reduced pressure is filtered. The obtained solid was dissolved completely by adding ethanol (50 mL), and crystallized by adding toluene (100 mL). After the crystallization was completed, it was filtered and dried under reduced pressure to obtain miglitol with a yield of 97.5% and a purity of 99.87%.
Embodiment 2
[0034] In the autoclave, SM-1 (10.00g, 44.80mmol), Pd / C (0.8g), and acetic acid (5.92g, 98.56mmol) were added to the tetrahydrofuran / purified water system (V: 四氢呋喃 :V: 纯化水 =1:2, 500mL), stirring, temperature control 35 ~ 40 ° C, pressure control at 6 ± 0.5Mpa reaction 6h, the reaction is completed, the reaction solution is cooled to room temperature, and the solid concentrated under reduced pressure is filtered. The obtained solid was dissolved completely by adding ethanol (50 mL), and crystallized by adding toluene (100 mL). After the crystallization was completed, it was filtered and dried under reduced pressure to obtain miglitol with a yield of 95.6% and a purity of 99.97%.
Embodiment 3
[0036] In the autoclave, SM-1 (10.00g, 44.80mmol), Pd / C (0.2g), and acetic acid (4.84g, 80.60mmol) were added to the tetrahydrofuran / purified water system (V: 四氢呋喃 :V: 纯化水 =1:2, 500mL), stirring, temperature control 35 ~ 40 ° C, pressure control at 6 ± 0.5Mpa reaction 6h, the reaction is completed, the reaction solution is cooled to room temperature, and the solid concentrated under reduced pressure is filtered. The obtained solid was dissolved completely by adding ethanol (50 mL), and crystallized by adding toluene (100 mL). After the crystallization was completed, it was filtered and dried under reduced pressure to obtain miglitol with a yield of 95.5% and a purity of 99.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com