Recombinant adeno-associated virus, preparation method thereof and application thereof in antibody detection
A virus vector and virus technology, applied in the field of recombinant adeno-associated virus and its preparation, can solve the problem of incomplete detection of adeno-associated virus antibodies in serum, inability to meet multiple uses of recombinant adeno-associated virus, and affect rAAV-mediated exogenous Efficiency and other issues, to achieve reliable marking effect, wide application value, and convenient collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a method for preparing recombinant adeno-associated virus, comprising the following steps:
[0039] (1) Preparation of clones capable of simultaneously expressing luciferase and green fluorescent protein
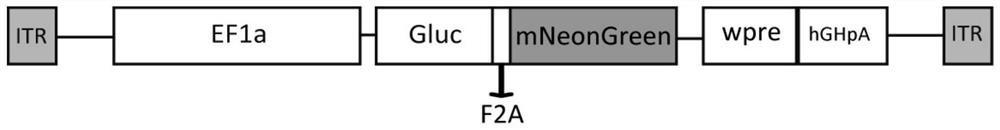
[0040] Using homologous recombination, SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 were sequentially inserted into the core skeleton of the adeno-associated virus vector. The vector was obtained from the Addgene plasmid library, and the vector number was Addgene#20298 , the obtained clone was named pAAV-EF1α-Gluc-2A-mNeonGreen; where SEQ ID NO.1 and SEQ ID NO.3 are the nucleotides of luciferase Gaussia luciferase and green fluorescent protein mNeonGreen after individual base substitutions, respectively sequence. Although the final translated amino acid sequence was the same as before the substitution, the expression of luciferase and green fluorescent protein was stronger after the base substitution. The luciferase expressed in the present invent...
Embodiment 1
[0048] Example 1 Preparation of a recombinant adeno-associated virus expressing luciferase and green fluorescent protein simultaneously
[0049] 1. Preparation of clones capable of simultaneously expressing luciferase and green fluorescent protein
[0050] First, the Gaussia Luciferase gene (SEQ ID NO.1) was synthesized by whole gene synthesis and inserted into the vector pUC57 to obtain pUC57-Gaussia Luciferase, and the fluorescent protein gene mNeonGreen (SEQ ID NO.3) was synthesized and inserted into the vector pUC57 to obtain To obtain pUC57-mNeonGreen, sequentially insert SEQ ID NO.1, SEQ ID NO.2 (2A gene) and SEQ ID NO.3 into the vector AAV core skeleton (Addgene #20298) by means of homologous recombination, and the obtained clone was named pAAV-EF1α-Gluc-2A-mNeonGreen;
[0051] The primers for amplifying the corresponding sequence are respectively as follows: the primers of the DNA fragment Gaussia Luciferase: SEQ ID NO:4 and SEQ ID NO:5, and the template is pUC57-mNeo...
Embodiment 2
[0056] Example 2 Detection of luciferase activity of recombinant adeno-associated virus expressing luciferase and green fluorescent protein simultaneously
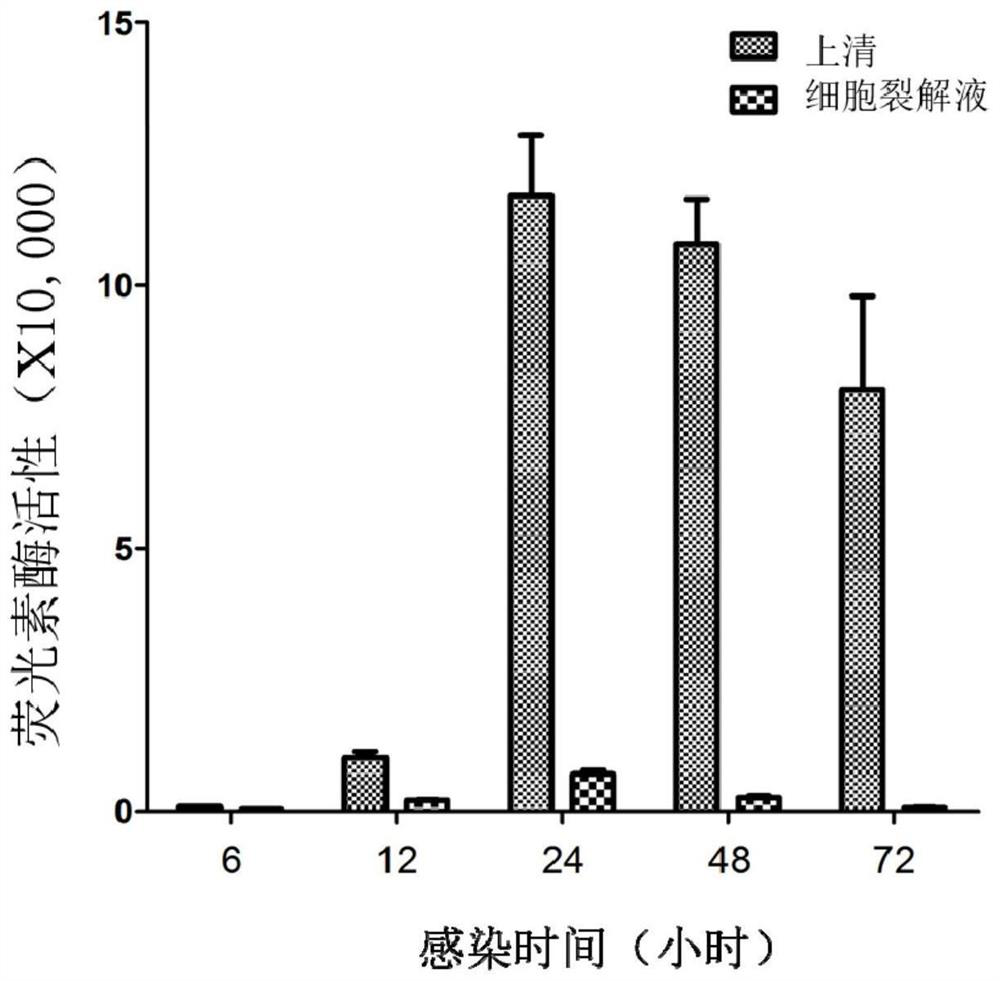
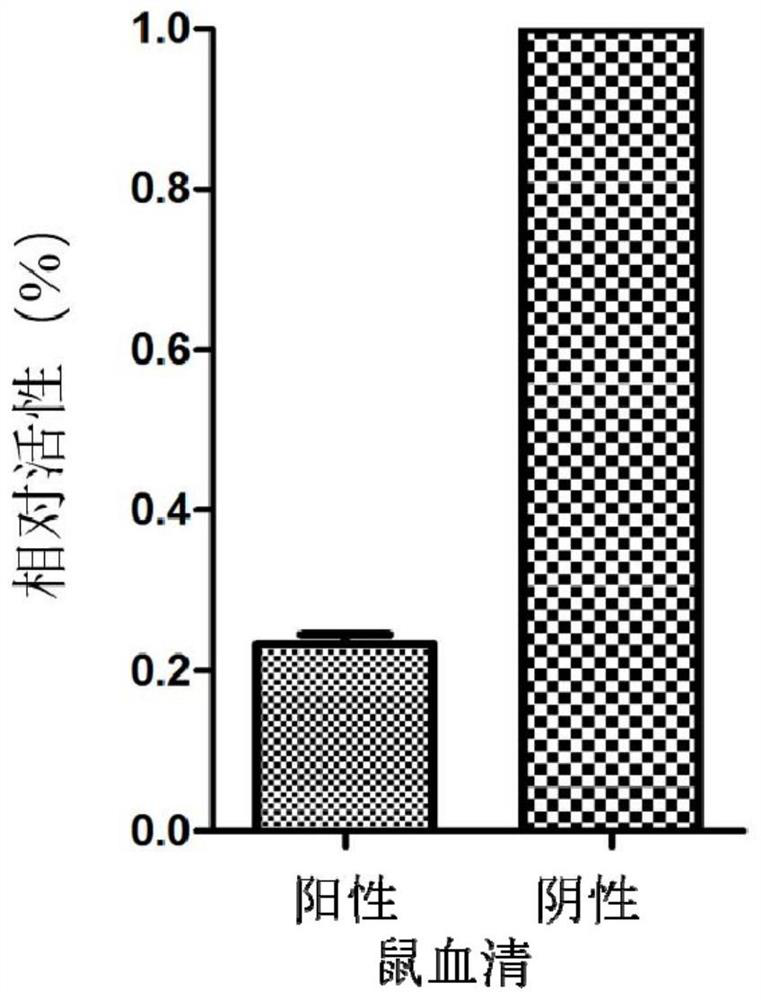
[0057] According to the ratio of 1:100, the recombinant virus was diluted with PBS, and 80 μL of the virus was taken to infect 293T cells (96-well plate), at 37 ° C, 5% CO 2 After culturing, the supernatant and cell lysate were collected respectively after 6, 12, 24, 48 and 72 hours after infection, and the activity of luciferase was detected, and the results were as follows: figure 2 shown. The results showed that: (1) the activity of luciferase could be detected 6 hours after infection, and its activity gradually increased with the prolongation of time, reaching the peak value at 24 hours after infection, and then the activity showed a downward trend; (2) ) the luciferase activity in the supernatant was significantly higher than that in the cell lysate. This result provides effective support for the subsequent detecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com