A kind of multi-antigen epitope protein of chicken infectious bursal disease virus and its application
A technology for bursal disease and chicken infectivity, applied in the direction of virus antigen components, viruses, virus peptides, etc., can solve the problems of urgent research on new vaccines, the inability of antibodies to resist viruses, and easy mutations, etc., to achieve inhibition and resistance to immune escape strains , control virus infection, low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
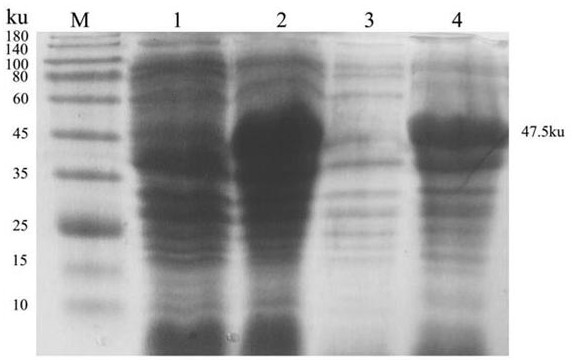
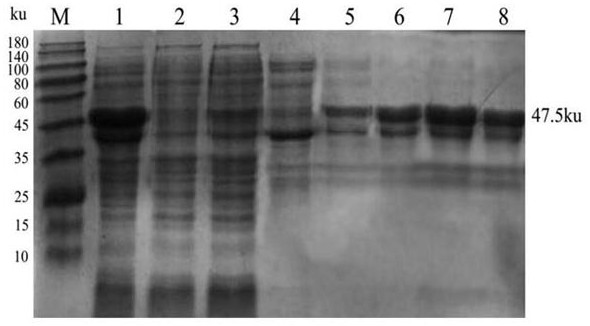
[0018] 1. Preparation of multi-antigen epitope fusion protein of infectious bursal disease virus
[0019] (1) Prediction and screening of epitopes of infectious bursal disease virus
[0020] Using the ABCpred prediction method combined with the DNAStar software Protean program to predict the epitopes of the VP2 and VP3 proteins of the IBDV reference strains HLJ0504 (GQ451330), Gx (AY444873), YS07 (FJ695138), and E (AF133904) in the GenBank database, Several B-cell antigenic sites and T-cell antigenic sites were screened by combining the two algorithms. The relevant information is shown in Table 1.
[0021] Table 1 Antigen sites related to IBDV multi-epitope protein
[0022]
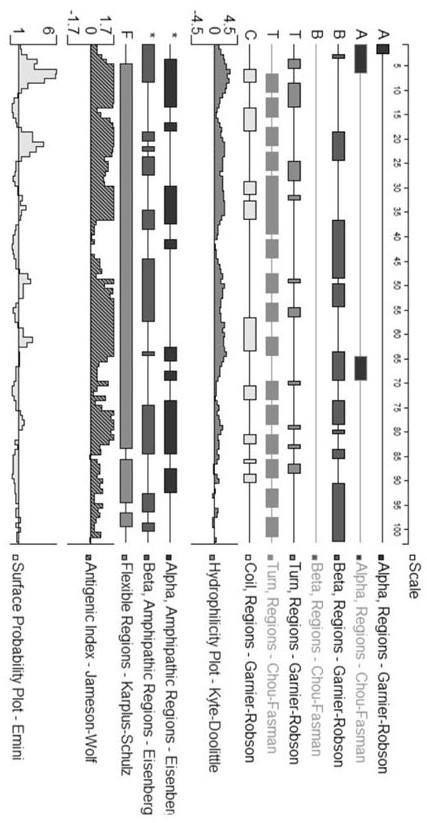
[0023] The amino acid sequence of the designed multi-antigen epitope protein was analyzed by Protean software, and the results were as follows: figure 1 , the rearranged multi-epitope protein has a higher antigenic index and a higher surface possibility, and can be used for prokaryotic expression to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com